Abstract
Fertility is a couple concept that has been measured since the beginning of demography, and male fecundity (his biological capacity to reproduce) is a component of the fertility rate. Unfortunately, we have no way of measuring the male component directly, although several indirect markers can be used. Population registers can be used to monitor the proportion of childless couples, couples who receive donor semen, trends in dizygotic twinning, and infertility diagnoses. Studies using time-to-pregnancy (TTP) may identify couple subfecundity, and TTP data will correlate with sperm quality and quantity as well as sexual activity and a number of other conditions. Having exposure data available for couples with a fecund female partner would make TTP studies of interest in identifying exposures that may affect male fecundity. Biological indicators such as sperm quality and quantity isolate the male component of fertility, and semen data therefore remain an important source of information for research. Unfortunately, often over half of those invited to provide a sperm sample will refuse, and the study is then subject to a selection that may introduce bias. Because the most important time windows for exposures that impair semen production could be early fetal life, puberty, and the time of ejaculation; longitudinal data over decades of time are required. The ongoing monitoring of semen quality and quantity should continue, and surveys monitoring fertility and waiting TTP should also be designed.
Keywords: data collection, epidemiology, epidemiological monitoring, fertility, sperm count, time-to-pregnancy (TTP)
INTRODUCTION
Fertility has been measured since the beginning of demography. Measuring fecundity, the biological capacity to reproduce, has a much shorter history, and causal infertility research has been long neglected, most likely due to the fear of global overpopulation. More research funds have been used to produce contraceptive methods that are safe and effective, and this research has, to a large extent, been successful. Perhaps the time has come to focus on how we can ensure that couples can have a child when they desire and, under optimal circumstances, without infertility treatment.
The fertility rate is a couple concept that measures how many children (or girls) women will have during the ages of reproduction (often 15–45 years), divided by the number of women in that age range in the population. A replacement rate of 2.1 children per woman in industrialized countries is necessary to maintain current population levels, and lower rates will lead to a decline in population size unless compensated by immigration and/or prolonged life expectancies. It has often been overlooked that the formula for the fertility rate based on cross-sectional data requires steady state conditions. If women postpone reproduction without altering their desire for a given family size, the fertility rate produces a biased low prediction of the actual fertility until a new steady-state condition is obtained with a new maternal age distribution of childbirth.1 Fertility rates in the oldest part of the age range of 15–45 years will be low until women who postpone their pregnancy reach that age. Countries that undergo changes in work and educational traditions for women will often see fertility rates decrease, and as a result, the number of children women will actually have is initially underestimated.
Male fertility is a component of the fertility rate and is partially driven by semen quality in addition to the male's role in shaping the desire for a given family size. Because the components of family planning change over time and because most of these components have little to do with male fecundity, changes in fertility are in general a poor marker of male fecundity. Considering changes in demographic fertility to reflect changes in male (or female) fecundity is seldom justified and always risky.2
OTHER REGISTER-BASED DEMOGRAPHIC MARKERS OF MALE FECUNDITY
In countries with good population registers, data on the proportion of women who have lived with a male partner during their adult lives and remain childless at the age of 45 years can be added to the monitoring of fertility rates. Many factors unrelated to male subfecundity could have played roles in the childlessness of these women,1 but an increasing trend would be a warning signal. The same is true for an increasing trend in women who have a male partner but seek insemination, in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) with donor semen. Trends will be influenced by the availability and affordability of these methods, but male subfecundity would also play a role. Trends in treatment for male infertility in general are a marker of male fecundity that should be considered, although such trends could also be caused by changes in health-seeking behavior over time. It is known that many infertile couples do not seek medical help.3,4
Trends in dizygotic twinning in couples who do not receive treatment for infertility may be another marker of male fecundity.5,6,7 Two eggs have to be fertilized within a short time period, which should be related to sperm quality and sexual activity. Nonetheless, dizygotic twinning is also an indicator influenced by many factors other than male fecundity, and we have only limited documentation for its quality as a male fecundity marker. Dizygotic twinning rates increase with age, indicating that other factors also play a role. Not all dizygotic twins represent high fecundity.
We Have No Direct Measure of Male Fecundity
Unfortunately, there is no measure of the male component of couple fecundity; only indirect indicators are available. Fertility is largely the result of two partners of the opposite sex attempting to have a child. Both partners have to be fecund to achieve success in untreated conditions, and they have to have sexual intercourse within the rather short fertile period of the menstrual cycle (4–6 days).8 Further, they must also stop using contraceptive methods.9 For these reasons, no systematic monitoring of fertility measures claim to monitor male fecundity.
The Standardized Fertility Ratio
Because many personal factors play a role in the decision to increase family size, higher internal validity could be obtained in studies attempting to identify causes of subfecundity using the case-only study principle, that is, letting the participants be their own control by using the experience from at least two different time windows. The SFR study, first presented by Levine et al.10 is such a model, in which observed fertility and expected fertility are compared before and after an exposure that may impact fecundity. Take for example, a group of workers who move to a different work process that may impair sperm quality or sexual desire/ability. In this design, the actual couple fertility before the exposure is compared with their expected fertility based on couples in their region, considering for example social class and age. This observed/expected SFR before exposure can now be compared with a similar SFR after exposure. If exposure has no impact on fecundity (the null hypothesis), the ratio of the SFRs should be 1.
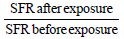
If the ratio of the SFRs is less than one, the relative fertility (adjusted for expected fertility) declined after the exposure. Although the decline could have resulted from the exposure, other factors may also play a role. For example, the unexposed group could have improved their fertility or the exposure may have correlates that interfere with fertility planning (e.g. changes in income, different working hours). These concerns make the design “weak” but not without interest. This design was able to identify changes in sperm quality following dibromochloropropane (DBCP) exposure,11,12 and changes were identified using a modified method by Starr and Levine.13 Even so, as a general survey instrument, it has limited value.
Case-Control Studies
Other fertility measures may also be used, such as diagnoses from fertility clinics or hospitals. In most cases, couples seeking treatment for infertility will undergo diagnostic examinations that include the male. These clinical data may be of value in identifying causes of male infertility using a case-control approach and a definition of the study base driven by the male patients.14 These data are, however, of limited interest in monitoring trends over time due to changes in seeking medical help and because only a limited number of individuals with fertility issues seek medical help.3
A case-control design including infertile couples that compares males with a partner who had no clinical reasons for infertility with males who did not have abnormal semen quality or quantity and who had a partner with a medically identified fertility problem may be used to identify risk factors for male subfecundity. Most other traditional case-control designs have otherwise limited interest because many subfecund men seek no treatment or are unaware of their problem. For this reason, the study base is defined by the patients who actually sought help, as it is performed when controls are selected from infertile couples under medical treatment.
Time-To-Pregnancy Data can also be Used as a Marker of Male Fecundity
Studies using TTP data have been widely used to identify couple fecundity because TTP data correlate with sperm quality and quantity as well as sexual activity.12,15,16 Most of these studies have used pregnancy cohorts (excluding sterile couples who seek treatment) or have been cross-sectional studies aimed at reconstructing the reproductive history of those selected, including their waiting TTP or the duration of unsuccessfully attempting to become pregnant. Longitudinal studies following couples trying to conceive have typically based their analytical models on exposures recorded from cycle to cycle while attempting to become pregnant.17 The proper time period of interest for the male is 72 days prior to the menstrual cycle under study or the time period of organogenesis and Sertoli cell production very early in life.18 Having exposure data available for couples with a fecund female partner would aid the ability of TTP studies to identify exposures that affect TTP via male fecundity. Studies of this type have not been conducted to monitor changes in male fecundity, most likely because using data on semen quality and quantity directly may appear more attractive.
However, semen studies often have much lower participation rates than TTP studies, and TTP studies should therefore remain in the tool box for studies of male fecundity.19
Biological Indicators
Semen quality and quantity are the most frequently used biological indicators of male fecundity, and they provide an opportunity to examine several semen characteristics, including total sperm count, semen concentration, sperm morphology, and sperm mobility. The indicator that is the “best” at predicting a conception is difficult to study because a sample of the semen that actually fertilizes the egg is seldom available; however, semen concentration and sperm morphology may be of higher value in pregnancy prediction than semen volume and sperm motility.17 Using semen donors who have been used successfully many times may be a more direct source of information. Serum biomarkers of spermatogenesis are easier to obtain than semen samples for testing biomarkers of spermatogenesis, and serum markers are also widely used in epidemiological studies. The serum levels of the gonadotropins follicle stimulating hormone (FSH) and luteinizing hormone (LH) are inversely associated with sperm concentration, morphology, and motility;20,21,22 whereas, inhibin B is positively associated with sperm concentration.20,21,22,23 However, these biomarkers are not superior to sperm concentration in predicting a conception;23 and therefore, they cannot replace semen analysis. Other indirect markers of male fecundity such as testis size, cryptorchidism, and anoscrotal distance have also been used.24,25,26,27
Data on secular trends in sperm counts or sperm concentration were reported by Carlsen et al. in the BMJ in 1992.28 Many other papers on trends in semen concentration over time were published both before and after; but the Carlsen paper received the most attention, perhaps because they provided a linear regression line showing a trend toward a situation with no sperm production. Because the plots used to create the regression line were obtained from different populations and were based on different sampling strategies, response rates, laboratory analyses, collecting strategies, etc.; most of the basic rules for comparing data over time were violated. Furthermore, a simple visual inspection of the plots suggests two parallel horizontal lines, with the upper level representing older data and a lower level representing more recent data. Had the data been presented with these two horizontal lines, the entire interpretation would have been different. It is also of concern that using median rather than mean sperm concentrations provides no clearly declining trends.29 One should therefore be cautious in not overinterpreting the results, although some take a declining sperm concentration as a fact.30 In a recent review by Sharpe,30 the BMJ figure was reproduced with only the regression line!
If there is a decline in sperm production and quality over time, it is a serious problem, and we need to determine its cause. At approximately the same time that Carlsen et al.28 published their paper, Sharpe and Skakkebaek31 provided a plausible environmental explanation for the decline. An increasing exposure to hormonal disruptors with estrogenic effects could be the culprit, but research following this paper only provided limited support to this hypothesis.32 Some studies suggest that prenatal exposure to diethylstilbestrol (DES) is associated with reduced sperm counts,33,34 but others do not,35,36 and Wilcox et al.37 showed no apparent effect on fecundity in men born to mothers who took DES while pregnant.
Monitoring comparable samples from young males drafted for military service over time using identical laboratory techniques has shown no or limited changes in recent years;38 perhaps even with a slight increase in semen concentration, which may be reassuring or indicate that we are now all being exposed to sperm-reducing factors that result in unusually low semen quality. Approximately 15% of couples who attempt to become pregnant will not succeed within the 1st year of trying, and some will not succeed even with medical help.39 Approximately 40% of males will have sperm counts that affect the waiting TTP.17,38 These figures are disturbing and indicate that human fecundity is lower than that observed for most animals.30
Unfortunately, semen samples, and therefore semen data, are difficult to obtain, and the samples may be subject to a selection that introduces bias. Men who believe they have a fertility problem may be more interested in taking part.40 For that reason, semen data should be sampled among young men who have not attempted to have offspring and therefore have no fertility information to base their consent on. Unfortunately, we do not know how well sperm data correlate between a young age and a later age. A Danish study on 158 young men (mean age 19 years at baseline) did not, however, observe a significant change in sperm quality or quantity during a 4-year follow-up period.41 However, advanced age is associated with a decline in semen quality and quantity.42 Although sperm production is determined by the number of Sertoli cells produced early in life (including fetal life)43 and the ejaculation rate in adult life, we do not know how stable the Sertoli cell population is throughout adulthood. Some exposures modify sperm production in adulthood (e.g., obesity,44 tobacco smoking,45 and pesticides such as DBCP, high doses of dichlorodiphenyltrichloroethane (DDT) and ethylene dibromide),46 although the most important causal window could begin shortly after conception.47,48,49 Because sperm count values heavily depend on sexual activity, a secular decline in sperm count could also reflect increasing sexual activity over time, perhaps as a function of better contraceptive means, shorter working hours, etc.50,51
What can be Done?
Almost all attention in male fertility research has been devoted to a few hypotheses52 inspired by insufficient data. The time has come to take a broader view on what may be affecting semen production and semen quality. In view of the lack of good evidence indicating the best semen marker of male fecundity, we should perhaps shift the focus from semen concentration to other characteristics of semen quality and quantity that are less vulnerable to the duration of abstinence. The focus should be on avoidable causes, but it could also include gene-environment interactions. Exposures of interest include dietary factors, infections, medication, vitamins, and environmental exposures, among others, at different time points.
While the epidemiologists wait for the next round of research opportunities for studying the potential perinatal etiology of sperm production, the ongoing monitoring of semen quality and quantity should continue.53 If semen production has decreased by more than 50% since the 1940s,28 we should examine changes in common lifestyle factors, new types of medication that are used frequently during pregnancy (such as acetaminophen) or changes in environmental exposures that affect many and began decades ago. Genetic forces of selection may operate more efficiently for a fertility trait than many other traits because the introduction of contraception and more recently assisted reproductive technologies (ART) has reduced family size and made subfecund couples as fertile (in the demographic sense) as superfertile couples; almost all obtain their one to three wanted children, although some do so with ART support.
It would also be of interest to obtain comparable data on semen quality from different countries based on solid and scientifically justified sampling and measurement criteria. The very limited data we have at present that can be used to make international comparisons indicate that the levels of semen quality and quantity vary among countries.54 A clear way to proceed is to follow emigrants to observe whether they develop the semen values of their new areas of residence, and if they do, how long the change requires.
Some studies could benefit from using stronger designs, such as the crossover design,55 sibling designs, and even twin studies.56 In the crossover design, the person is his own control because two semen samples are compared over a time period when exposure during sperm production has changed. In the sibling design, one can also examine the effect on sperm production of exposure that changed from one pregnancy to the next.
Studies monitoring fertility and waiting TTP should also be designed.19 After all, the best indication of semen quality is a partner's pregnancy and the TTP. At the population level, this indicator will correlate with semen quality, when all other factors are held equal. It is not easy to conduct these studies, but we now know more about how to do so than we did 10 years ago.38
QUESTIONS FROM THE PANEL
Q1: Key characteristics of studies monitoring fertility and waiting time to pregnancy
A1: Both fertility and waiting TTP have many determinants that make simple monitoring difficult to interpret. We know that fertility to a large extent reflects social and educational changes in a population. However, access to contraceptive methods and induced abortion also plays a role in addition to biological fecundity. Because the preferred family size is small in most affluent societies where women take an active part in education and work, most couples will be able to reach their fertility goal even if one or both suffer from subfecundity. The waiting TTP (or to a successful pregnancy resulting in a birth) may reflect potential changes in fecundity over time or between populations. These measures must, however, be observed in a context that considers changes over time in other component causes. To adjust for these factors, monitoring should provide some information on these variables.
To monitor waiting TTP, a validated questionnaire should be developed. Several measurements rely on how questions are phrased, and the results need not be comparable if studies are based on different questions. A key question is whether the pregnancy was planned or not-only planned pregnancies have a waiting TTP. The history of contraceptive use and sexual activity is also important. The use of safe contraceptive methods allows pregnancy planning, whereas the use of unsafe methods leads to unplanned pregnancies. Social conditions, family structure and job conditions, among other factors, play roles in family planning and the willingness to pursue a pregnancy. Monitoring based on how many individuals have a TTP of 12 months or more requires that few give up after, for example, 6 months of trying. Access to infertility treatment, age and parity also play a major role. At best, a monitoring system should cover the entire fertile time period from 15 to 45 years of age. Nationwide monitoring of the female population is not necessary, but large, randomly selected groups should complete a questionnaire at regular intervals, and a high participation rate is crucial to avoid selection bias. Social changes will move pregnancy planning forward or backward in time, and the outcomes of pregnancies play a role in avoiding pregnancy or trying to become pregnant. A simple monitoring system could address women when they reach 45 years of age, with the hope that they will recall their reproductive experience, including waiting times of 12 months or more, regardless of whether they were successful. Another very simple monitoring system would be to ask pregnant women about their TTP for the current pregnancy and let this be part of the reporting to a medical birth register. Despite all the limitations of such a system, its simplicity is attractive, and it could be a useful inspiration for targeted studies. Most factors affecting fecundity will also change the TTP distribution. Few, if any, factors are expected to have an “all or none” effect.
When monitoring sperm counts in adult life, a number of factors must be considered in addition to the risk of selection bias. The most important of these is sexual activity.57,58 Most studies have attempted to implement semen sampling following at least 2-7 days of sexual abstinence in accordance with the World Health Organization (WHO) laboratory manual for the examination and processing of human semen,59 but we know that this rule is often violated and that it may be much too imprecise.60 Most samples are obtained through masturbation, and the conditions around this sampling as well as transportation time and the conditions during transportation can affect the sample. More strict rules for the laboratory techniques have only recently been implemented, but measurement errors remain frequent, and the variance over time or between laboratories may be substantial.61,62
Because the most important time window for exposure that impairs semen production could be from early fetal life to early childhood,43 longitudinal data are required over decades to identify causes that operate early in life. It is difficult for study subjects to recall events that occurred many years ago, although pregnancy is a time period mothers will remember better than most other time periods. The mother may recall smoking, breastfeeding, and an occupational history for a pregnancy that occurred 20–40 years ago, but she will not remember medication, dietary factors, minor infections, or many other exposures of interest. These data will have to be collected in real time as will data regarding biomarkers of pollutants, vitamins, etc., The next important round of semen studies will be conducted when the boys from our original pregnancy cohort become 18 years or older and are ready to be invited to provide semen samples for research. We have observed small studies on maternal smoking, alcohol intake, and obesity as well as serum concentrations of perfluorochemicals (PFCs) during pregnancy.47,48,63,64,65 In a few years, we can repeat these studies with much larger samples and also examine maternal medication and diseases during pregnancy that could impact Sertoli cell production in sons.
In short, when monitoring a given condition to determine whether it changes over time, the only factor that should vary over time is time, or the factor that affects the measure of interest should be controllable over time. Therefore, we have to record its determinants and adjust for them in the analysis.
Q2: Evidence to suggest that male infertility is a developmental disease
A2: With the exception of a few findings, the evidence to support the hypothesis that male infertility is a developmental disease is speculative and sparse. Cryptorchidism (undescended testis) is associated with lower sperm counts and lower paternity rates, particularly if not corrected by surgery at a young age.66,67 It has been hypothesized that poor semen quality, testicular cancer, cryptorchidism, and hypospadias (an abnormally placed urinary meatus) are symptoms of one underlying entity originating from fetal life — the so-called testicular dysgenesis syndrome (TDS).68 However, most data related to pathophysiology as well as data from epidemiological studies are not strongly in accordance with the TDS hypothesis.67,69 Chromosomal disorders, such as Klinefelter syndrome, which occurs in approximately 1 in 600 male births, are related to poor semen quality and quantity as well as sexual manifestation.70,71
Some data on pre- or perinatal exposures correlate with low sperm counts in the offspring and prolonged waiting times to pregnancy, but there are few two-generation studies. There is good reason to believe that the early phase of life, including organ development and differentiation, is of importance, particularly regarding male fecundity.43 Low birth weight is a risk factor for testicular cancer,72 cryptorchidism,73 and hypospadias;74 whereas, the association between birth weight and semen quality is less clear.75,76,77 Twin studies show that not all cases of potential late growth restriction or preterm birth are associated with poor sperm data.56 Twins have normal sperm counts. Genes and epigenetic factors may play important roles in male fertility because a growing body of evidence suggests that environmental exposures (nutritional, chemical, and physical factors) have the potential to change the epigenome and thereby alter gene expression, resulting in a modified disease susceptibility.78 Mumps, other infections, and trauma are also known to affect the testes. It is therefore likely that sperm health should be viewed in a life-course perspective, where exposures at different developmental stages play a role. Poor sperm count as well as testicular cancer may require several “hits” to become a manifest health problem, and these “hits” may occur throughout the life of an individual.
We do not believe that it is justified at present to label low sperm count a congenital disorder, except in cases of early malplacement or malfunction of the testes. After all, sperm production continues throughout life, although at a reduced intensity with age. At present, it is wise to keep an open mind concerning the timing of exposure in studies on sperm counts. Additionally, if possible, the pre- and perinatal time slot should remain open for investigation.
In short, common sense indicates that poor fecundity is partially a developmental disorder. The time period of organ development is also a time period of relevance for later organ functioning.
CONCLUSIONS
Reproduction is a couple concept, and the health of spermatozoa therefore deserves public health interest. Threats to the health of spermatozoa should be identified, reduced, and if possible, removed.
Most of our research experience comes from a rather limited time period, and too much hype surrounding not well-documented changes in fertility may have tired the public and funding agencies, which is a pity. Although the world's population is still growing, this will come to an end with better education, better economic means; and thus, less religious fanaticism. Current social secular trends postpone reproduction until late in life, which may reduce the window of opportunity to reach the desired family size to a too short time period, at least for families with a fecundity problem for the man, the woman or both.
Many observations indicate that fertility research should be observed in a life-course perspective and that prevention should start before conception. Subfecundity for both men and women may well be a transgenerational effect involving the development of the male genitalia.
We have sufficient evidence to state that many men have poor semen quality and quantity and that they may not be able to father a child or obtain the number of children they want–at least not without medical help. Whether these problems are new or increasing is less well-known, but the problems cannot be disregarded. For these reasons, we need to continue our search for male fertility risk factors. We must also continue monitoring trends over time.
COMPETING INTERESTS
All authors declare that they have no competing interests.
REFERENCES
- 1.Schmidt L, Sobotka T, Bentzen JG, Nyboe Andersen A. ESHRE Reproduction and Society Task Force. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update. 2012;18:29–43. doi: 10.1093/humupd/dmr040. [DOI] [PubMed] [Google Scholar]
- 2.Mills M, Rindfuss RR, McDonald P, te Velde E. ESHRE Reproduction and Society Task Force. Why do people postpone parenthood?. Reasons and social policy incentives. Hum Reprod Update. 2011;17:848–60. doi: 10.1093/humupd/dmr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt L. Infertility and assisted reproduction in Denmark. Epidemiology and psychosocial consequences. Dan Med Bull. 2006;53:390–417. [PubMed] [Google Scholar]
- 4.Olsen J, Kuppers-Chinnow M, Spinelli A. Seeking medical help for subfecundity: a study based upon surveys in five European countries. Fertil Steril. 1996;66:95–100. [PubMed] [Google Scholar]
- 5.Derom C, Gielen M, Peeters H, Frijns JP, Zeegers MP. Time trends in the natural dizygotic twinning rate. Hum Reprod. 2011;26:2247–52. doi: 10.1093/humrep/der180. [DOI] [PubMed] [Google Scholar]
- 6.Zhu JL, Basso O, Obel C, Christensen K, Olsen J. Danish National Birth Cohort. Infertility, infertility treatment and twinning: the Danish National Birth Cohort. Hum Reprod. 2007;22:1086–90. doi: 10.1093/humrep/del495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James WH. Monitoring reproductive health in Europe: what are the best indicators? Hum Reprod. 2007;22:1197–9. doi: 10.1093/humrep/dem020. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333:1517–21. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox AJ, Dunson DB, Weinberg CR, Trussell J, Baird DD. Likelihood of conception with a single act of intercourse: providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63:211–5. doi: 10.1016/s0010-7824(01)00191-3. [DOI] [PubMed] [Google Scholar]
- 10.Levine RJ, Symons MJ, Balogh SA, Milby TH, Whorton MD. A method for monitoring the fertility of workers. 2. Validation of the method among workers exposed to dibromochloropropane. J Occup Med. 1981;23:183–8. [PubMed] [Google Scholar]
- 11.Levine RJ, Blunden PB, DalCorso RD, Starr TB, Ross CE. Superiority of reproductive histories to sperm counts in detecting infertility at a dibromochloropropane manufacturing plant. J Occup Med. 1983;25:591–7. [PubMed] [Google Scholar]
- 12.Levine RJ. Monitoring fertility to detect toxicity to the male reproductive system. Reprod Toxicol. 1988;2:223–7. doi: 10.1016/0890-6238(88)90027-5. [DOI] [PubMed] [Google Scholar]
- 13.Starr TB, Levine RJ. Assessing effects of occupational exposure on fertility with indirect standardization. Am J Epidemiol. 1983;118:897–904. doi: 10.1093/oxfordjournals.aje.a113707. [DOI] [PubMed] [Google Scholar]
- 14.Ramlau-Hansen CH, Stoltenberg CD, Hougaard KS, Parner ET, Toft G, et al. MINERVA-group. Male-mediated infertility in sons of building painters and gardeners: a nationwide register-based follow-up study. Reprod Toxicol. 2012;34:522–8. doi: 10.1016/j.reprotox.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg CR, Baird DD, Wilcox AJ. Sources of bias in studies of time to pregnancy. Stat Med. 1994;13:671–81. doi: 10.1002/sim.4780130528. [DOI] [PubMed] [Google Scholar]
- 16.Rachootin P, Olsen J. The risk of infertility and delayed conception associated with exposures in the Danish workplace. J Occup Med. 1983;25:394–402. [PubMed] [Google Scholar]
- 17.Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 18.Campion S, Catlin N, Heger N, McDonnell EV, Pacheco SE, et al. Male reprotoxicity and endocrine disruption. EXS. 2012;101:315–60. doi: 10.1007/978-3-7643-8340-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen J, Rachootin P. Invited commentary: monitoring fecundity over time--if we do it, then let's do it right. Am J Epidemiol. 2003;157:94–7. doi: 10.1093/aje/kwf178. [DOI] [PubMed] [Google Scholar]
- 20.Jensen TK, Andersson AM, Hjollund NH, Scheike T, Kolstad H, et al. Inhibin B as a serum marker of spermatogenesis: correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men. J Clin Endocrinol Metab. 1997;82:4059–63. doi: 10.1210/jcem.82.12.4456. [DOI] [PubMed] [Google Scholar]
- 21.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 22.Yalti S, Gurbuz B, Ficicioglu C. Serum levels of inhibin B in men and their relationship with gonadal hormones, testicular volume, testicular biopsy results and sperm parameters. J Obstet Gynaecol. 2002;22:649–54. doi: 10.1080/0144361021000020466. [DOI] [PubMed] [Google Scholar]
- 23.Mabeck LM, Jensen MS, Toft G, Thulstrup M, Andersson M, et al. Fecundability according to male serum inhibin B – a prospective study among first pregnancy planners. Hum Reprod. 2005;20:2909–15. doi: 10.1093/humrep/dei141. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberg ML, Jensen TK, Walters RC, Skakkebaek NE, Lipshultz LI. The relationship between anogenital distance and reproductive hormone levels in adult men. J Urol. 2012;187:594–8. doi: 10.1016/j.juro.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Sathyanarayana S, Beard L, Zhou C, Grady R. Measurement and correlates of ano-genital distance in healthy, newborn infants. Int J Androl. 2010;33:317–23. doi: 10.1111/j.1365-2605.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahk JY, Jung JH, Jin LM, Min SK. Cut-off value of testes volume in young adults and correlation among testes volume, body mass index, hormonal level, and seminal profiles. Urology. 2010;75:1318–23. doi: 10.1016/j.urology.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 27.van Brakel J, Kranse R, de Muinck Keizer-Schrama SM, Hendriks AE, de Jong FH, et al. Fertility potential in men with a history of congenital undescended testes: a long-term follow-up study. Andrology. 2013;1:100–8. doi: 10.1111/j.2047-2927.2012.00024.x. [DOI] [PubMed] [Google Scholar]
- 28.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieschlag E, Lerchl A. Sperm crisis: what crisis? Asian J Androl. 2012;15:184–6. doi: 10.1038/aja.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe RM. Sperm counts and fertility in men: a rocky road ahead. Science and Society Series on Sex and Science. EMBO Rep. 2012;13:398–403. doi: 10.1038/embor.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–5. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 32.Storgaard L, Bonde JP, Olsen J. Male reproductive disorders in humans and prenatal indicators of estrogen exposure. A review of published epidemiological studies. Reprod Toxicol. 2006;21:4–15. doi: 10.1016/j.reprotox.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Gill WB, Schumacher GF, Bibbo M, Straus FH, 2nd, Schoenberg HW. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J Urol. 1979;122:36–9. doi: 10.1016/s0022-5347(17)56240-0. [DOI] [PubMed] [Google Scholar]
- 34.Stenchever MA, Williamson RA, Leonard J, Karp LE, Ley B, et al. Possible relationship between in utero diethylstilbestrol exposure and male fertility. Am J Obstet Gynecol. 1981;140:186–93. doi: 10.1016/0002-9378(81)90107-1. [DOI] [PubMed] [Google Scholar]
- 35.Andonian RW, Kessler R. Transplacental exposure to diethylstilbestrol in men. Urology. 1979;13:276–9. doi: 10.1016/0090-4295(79)90420-5. [DOI] [PubMed] [Google Scholar]
- 36.Leary FJ, Resseguie LJ, Kurland LT, O’Brien PC, Emslander RF, et al. Males exposed in utero to diethylstilbestrol. JAMA. 1984;252:2984–9. [PubMed] [Google Scholar]
- 37.Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL. Fertility in men exposed prenatally to diethylstilbestrol. N Engl J Med. 1995;332:1411–6. doi: 10.1056/NEJM199505253322104. [DOI] [PubMed] [Google Scholar]
- 38.Jørgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012:2. doi: 10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juul S, Karmaus W, Olsen J. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. The European Infertility and Subfecundity Study Group. Hum Reprod. 1999;14:1250–4. doi: 10.1093/humrep/14.5.1250. [DOI] [PubMed] [Google Scholar]
- 40.Larsen SB, Abell A, Bonde JP. Selection bias in occupational sperm studies. Am J Epidemiol. 1998;147:681–5. doi: 10.1093/oxfordjournals.aje.a009509. [DOI] [PubMed] [Google Scholar]
- 41.Carlsen E, Swan SH, Petersen JH, Skakkebaek NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod. 2005;20:942–9. doi: 10.1093/humrep/deh704. [DOI] [PubMed] [Google Scholar]
- 42.Auger J, Jouannet P. Age and male fertility: biological factors. Rev Epidemiol Sante Publique. 2005;53(Spec No 2):2S25–35. [PubMed] [Google Scholar]
- 43.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 44.Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221–31. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vine MF, Margolin BH, Morrison HI, Hulka BS. Cigarette smoking and sperm density: a meta-analysis. Fertil Steril. 1994;61:35–43. [PubMed] [Google Scholar]
- 46.Jurewicz J, Hanke W, Radwan M, Bonde JP. Environmental factors and semen quality. Int J Occup Med Environ Health. 2009;22:305–29. doi: 10.2478/v10001-009-0036-1. [DOI] [PubMed] [Google Scholar]
- 47.Ramlau-Hansen CH, Thulstrup AM, Storgaard L, Toft G, Olsen J, et al. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol. 2007;165:1372–9. doi: 10.1093/aje/kwm032. [DOI] [PubMed] [Google Scholar]
- 48.Ramlau-Hansen CH, Nohr EA, Thulstrup AM, Bonde JP, Storgaard L, et al. Is maternal obesity related to semen quality in the male offspring? A pilot study. Hum Reprod. 2007;22:2758–62. doi: 10.1093/humrep/dem219. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Wang H, Ji YL, Zhang Y, Yu T, et al. Maternal fenvalerate exposure during pregnancy persistently impairs testicular development and spermatogenesis in male offspring. Food Chem Toxicol. 2010;48:1160–9. doi: 10.1016/j.fct.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Olsen J, Zhu JL, Ramlau-Hansen CH. Has fertility declined in recent decades? Acta Obstet Gynecol Scand. 2011;90:129–35. doi: 10.1111/j.1600-0412.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- 51.Olsen J. Is human fecundity declining--and does occupational exposures play a role in such a decline if it exists? Scand J Work Environ Health. 1994;20(Spec No):72–7. [PubMed] [Google Scholar]
- 52.Safe S. Endocrine disruptors and falling sperm counts: lessons learned or not! Asian J Androl. 2013;15:191–4. doi: 10.1038/aja.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonde JP, Ramlau-Hansen CH, Olsen J. Trends in sperm counts: the saga continues. Epidemiology. 2011;22:617–9. doi: 10.1097/EDE.0b013e318223442c. [DOI] [PubMed] [Google Scholar]
- 54.Nordkap L, Joensen UN, Blomberg JM, Jorgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–30. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 55.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 56.Storgaard L, Bonde JP, Ernst E, Andersen CY, Spanô M, et al. Genetic and environmental correlates of semen quality: a twin study. Epidemiology. 2006;17:674–81. doi: 10.1097/01.ede.0000239730.47963.4e. [DOI] [PubMed] [Google Scholar]
- 57.Pellestor F, Girardet A, Andreo B. Effect of long abstinence periods on human sperm quality. Int J Fertil Menopausal Stud. 1994;39:278–82. [PubMed] [Google Scholar]
- 58.Håkonsen LB, Spano M, Bonde JP, Olsen J, Thulstrup AM, et al. Exposures that may affect sperm DNA integrity: two decades of follow-up in a pregnancy cohort. Reprod Toxicol. 2012;33:316–21. doi: 10.1016/j.reprotox.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 59.5th ed. Geneva: World Health Organization; 2010. World Health Organisation. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 60.Amann RP. Evaluating testis function non-invasively: how epidemiologist-andrologist teams might better approach this task. Hum Reprod. 2010;25:22–8. doi: 10.1093/humrep/dep383. [DOI] [PubMed] [Google Scholar]
- 61.Lu JC, Zhang HY, Hu YA, Huang YF, Lu NQ. A survey on the status of semen analysis in 118 laboratories in China. Asian J Androl. 2010;12:104–10. doi: 10.1038/aja.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mallidis C, Cooper TG, Hellenkemper B, Lablans M, Uckert F, et al. Ten years’ experience with an external quality control program for semen analysis. Fertil Steril. 2012;98:611–6.e4. doi: 10.1016/j.fertnstert.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Ramlau-Hansen CH, Toft G, Jensen MS, Strandberg-Larsen K, Hansen ML, et al. Maternal alcohol consumption during pregnancy and semen quality in the male offspring: two decades of follow-up. Hum Reprod. 2010;25:2340–5. doi: 10.1093/humrep/deq140. [DOI] [PubMed] [Google Scholar]
- 64.Cirillo PM, Cohn BA, Krigbaum NY, Lee M, Brazil C, et al. Effect of maternal coffee, smoking and drinking behavior on adult son's semen quality: prospective evidence from the Child Health and Development Studies. J DOHaD. 2011;2:375–86. doi: 10.1017/S2040174411000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vested A, Ramlau-Hansen CH, Olsen SF, Bonde JP, Kristensen SL, et al. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ Health Perspect. 2013;121:453–8. doi: 10.1289/ehp.1205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy F, Paran TS, Puri P. Orchidopexy and its impact on fertility. Pediatr Surg Int. 2007;23:625–32. doi: 10.1007/s00383-007-1900-3. [DOI] [PubMed] [Google Scholar]
- 67.Thorup J, McLachlan R, Cortes D, Nation TR, Balic A, et al. What is new in cryptorchidism and hypospadias – a critical review on the testicular dysgenesis hypothesis. J Pediatr Surg. 2010;45:2074–86. doi: 10.1016/j.jpedsurg.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 68.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 69.Akre O, Richiardi L. Does a testicular dysgenesis syndrome exist? Hum Reprod. 2009;24:2053–60. doi: 10.1093/humrep/dep174. [DOI] [PubMed] [Google Scholar]
- 70.Maiburg M, Repping S, Giltay J. The genetic origin of Klinefelter syndrome and its effect on spermatogenesis. Fertil Steril. 2012;98:253–60. doi: 10.1016/j.fertnstert.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 71.Harnisch B, Oates R. Genetic disorders related to male factor infertility and their adverse consequences. Semin Reprod Med. 2012;30:105–15. doi: 10.1055/s-0032-1307418. [DOI] [PubMed] [Google Scholar]
- 72.Cook MB, Akre O, Forman D, Madigan MP, Richiardi L, et al. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer – experiences of the son. Int J Epidemiol. 2010;39:1605–18. doi: 10.1093/ije/dyq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jensen MS, Wilcox AJ, Olsen J, Bonde JP, Thulstrup AM, et al. Cryptorchidism and hypospadias in a cohort of 934,538 Danish boys: the role of birth weight, gestational age, body dimensions, and fetal growth. Am J Epidemiol. 2012;175:917–25. doi: 10.1093/aje/kwr421. [DOI] [PubMed] [Google Scholar]
- 74.Stokowski LA. Hypospadias in the neonate. Adv Neonatal Care. 2004;4:206–15. doi: 10.1016/j.adnc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Francois I, de ZF, Spiessens C, D’Hooghe T, Vanderschueren D. Low birth weight and subsequent male subfertility. Pediatr Res. 1997;42:899–901. doi: 10.1203/00006450-199712000-00029. [DOI] [PubMed] [Google Scholar]
- 76.Olsen J, Bonde JP, Basso O, Hjollund NH, Sorensen HT, et al. Birthweight and semen characteristics. Int J Androl. 2000;23:230–5. doi: 10.1046/j.1365-2605.2000.00239.x. [DOI] [PubMed] [Google Scholar]
- 77.Ramlau-Hansen CH, Hansen M, Jensen CR, Olsen J, Bonde JP, et al. Semen quality and reproductive hormones according to birthweight and body mass index in childhood and adult life: two decades of follow-up. Fertil Steril. 2010;94:610–8. doi: 10.1016/j.fertnstert.2009.01.142. [DOI] [PubMed] [Google Scholar]
- 78.Dada R, Kumar M, Jesudasan R, Fernandez JL, Gosalvez J, et al. Epigenetics and its role in male infertility. J Assist Reprod Genet. 2012;29:213–23. doi: 10.1007/s10815-012-9715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


