Abstract
Over the last few decades, there have been numerous reports of adverse effects on the reproductive health of wildlife and laboratory animals caused by exposure to endocrine disrupting chemicals (EDCs). The increasing trends in human male reproductive disorders and the mounting evidence for causative environmental factors have therefore sparked growing interest in the health threat posed to humans by EDCs, which are substances in our food, environment and consumer items that interfere with hormone action, biosynthesis or metabolism, resulting in disrupted tissue homeostasis or reproductive function. The mechanisms of EDCs involve a wide array of actions and pathways. Examples include the estrogenic, androgenic, thyroid and retinoid pathways, in which the EDCs may act directly as agonists or antagonists, or indirectly via other nuclear receptors. Dioxins and dioxin-like EDCs exert their biological and toxicological actions through activation of the aryl hydrocarbon-receptor, which besides inducing transcription of detoxifying enzymes also regulates transcriptional activity of other nuclear receptors. There is increasing evidence that genetic predispositions may modify the susceptibility to adverse effects of toxic chemicals. In this review, potential consequences of hereditary predisposition and EDCs are discussed, with a special focus on the currently available publications on interactions between dioxin and androgen signaling.
Keywords: androgen receptor, aryl hydrocarbon receptor, endocrine disrupter
INTRODUCTION
In 1992, a meta-analysis indicated falling sperm counts in otherwise normal men over a 50-year period.1 At the same time, the incidence of testicular cancer had risen dramatically in some western countries.2 There are also reports on increasing frequency of genital malformations such as cryptorchidism and hypospadias in newborn boys.3
Since these trends have emerged over a relatively short time span, they have been proposed to be caused by exposure to environmental antiandrogens or lifestyle factors with adverse effects on the male reproductive system. In support of this, epidemiological studies on testicular cancer have shown that first generation Nordic immigrants to Sweden had a prevalence of testicular cancer similar to the one found in their country of origin, whereas their offspring, who were born and raised in Sweden, had a frequency of testicular cancer similar to the prevalence found in Swedish men.4 Ethnic differences in the incidence of reproductive disorders, such as cryptorchidism,5 hypospadias,6 testicular cancer7,8 and prostate cancer,9,10 illustrate that genetic components that contribute to the susceptibility are also operating. This is also observed in animal models, where certain rodent strains differ in their response to xenobiotics.11,12
It is increasingly recognized that the etiology of most common diseases involves not only discrete genetic and environmental factors, but also interactions between the two. Obvious gene-environment interactions include the much stronger effect of sunlight exposure on skin cancer risk in fair-skinned humans compared to dark-skinned individuals. Other well-described examples where gene-environment interactions affect the susceptibility to common human diseases include diabetes13 and cancer.14
Gene-environment interactions can be exploited to improve human health. A well-known example of this relates to a rare autosomal recessive mutation in the gene encoding the hepatic enzyme phenylalanine hydroxylase, which leads to the metabolic disease phenylketonuria. Under normal dietary conditions, carriers of the phenylketonuria mutation suffer severe impairment in cognitive development, but a phenylalanine-deficient diet substantially improves their prognosis. Another example is illustrated by the influence of smoking and alcohol intake on the impact of polymorphisms in the genes encoding apoE and alcohol dehydrogenase, respectively, on coronary heart disease (reviewed by Talmud15).
Identifying and validating gene-environment interactions will further increase our understanding of how specific environmental exposures cause disease by pinpointing genes through which the environmental effects are conveyed. Since mechanisms through which most endocrine disrupting chemicals (EDCs) affect reproductive health are largely unknown, it is of interest to investigate whether adverse environmental effects on reproductive health are modified by genetic predisposition. Among all EDCs, the mechanism through which dioxins and dioxin-like chemicals exert their biological and toxicological effects has been characterized best. Moreover, several crosstalk mechanisms between dioxin-induced signaling pathways and reproductive signaling pathways have been described. Therefore, this review aims to examine whether genetic variation in genes involved in dioxin-induced signaling contribute to an individual's susceptibility to the adverse effects of dioxins and dioxin-like EDCs on male reproductive health.
AROMATIC HYDROCARBONS
EDCs form a highly heterogeneous group of compounds, of which aromatic hydrocarbons, also known as arenes or aryl hydrocarbons, are among the most significant groups of persistent EDCs. Aromatic hydrocarbons can be monocyclic, e.g. benzene, furan and pyridine, or polycyclic, also called polyaromatic hydrocarbons (PAHs), with two to seven fused benzene rings, e.g. naphthalene and benzo[a]pyrene. Halogenated PAHs like 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and dioxin-like compounds such as some polychlorinated biphenyls (PCBs) are released from household sources as well as from industrial and natural emissions. Some of them, such as benzo[a]pyrene, also occur as by-products of cigarette smoking.
The aryl hydrocarbon receptor
Both PAHs and halogenated PAHs exert their biological and toxic effects through activation of the aryl hydrocarbon receptor (AHR), also known as the dioxin receptor. Although the human AHR was discovered almost 4 decades ago,16 its physiological role and endogenous ligand initially remained unknown and it was therefore designated as an orphan receptor. Several candidate activators have since been presented, including the heme metabolites indirubin and bilirubin,17,18 the arachidonic acid metabolite lipoxin 4A19 and most recently the tryptophan metabolite kynurenine.20,21
The AHR is evolutionary well-conserved and ubiquitously expressed in mammalian tissues.22 AHR knockout mice are characterized by liver fibrosis23 as well as hampered embryonic development of a wide range of organs,24,25 reduced xenobiotic metabolism, immune system defects26,27,28 and regulation of hematopoiesis.29 These animal studies also revealed that the AHR is essential in the reproductive system, for example for testosterone synthesis and sperm production30 as well as for normal prostate and seminal vesicle development.31 Knocking out the AHR gene in a prostate cancer mouse model (TRAMP) inhibited prostatic carcinogenesis, while treating TRAMP mice with an AHR modulator inhibited metastasis.32
Mice with constitutively active AHR on the other hand, had a reduced life span and spontaneously developed liver33 and stomach tumors.34 In three independent founder lines of the mice, heterozygous mice showed less severe stomach tumors than homozygous mice, indicating a gene-dosage effect. Furthermore, the severity of the gastric tumors increased with age, and males were affected more severely and died earlier than females further illustrating a sex difference in susceptibility to dioxin.
Molecular function of the AHR
The AHR is a ligand-activated transcription factor that belongs to a family of signal transduction proteins that contain a basic helix-loop-helix motif,35 which is a conserved region in many transcription factors, and in addition a Per/AHR nuclear translocator (ARNT)/Sim (PAS) domain. Based on sequence similarity, these basic helix-loop-helix/PAS proteins can be divided into two phylogenetic groups; the ARNT group containing: ARNT, ARNT2, ARNT3 and Per, and the AHR group containing: AHR, Sim and hypoxia-inducible factor 1α.36,37,38,39,40
The unliganded AHR resides in the cytosol associated with HSP90 and AHR-interacting protein (AIP), also known as hepatitis B virus X-associated protein (XAP2) or AHR activator 9 (ARA9). Upon ligand binding at the PAS domain, the receptor undergoes a conformational change and migrates to the nucleus, where it heterodimerizes with the ARNT protein41 and the complex subsequently interacts with consensus dioxin or xenobiotic responsive elements in enhancers/promoters of specific target genes including the genes for the enzymes CYP1A1 (cytochrome P450, subfamily I, polypeptide 1, also known as aryl hydrocarbon hydroxylase) and CYP1B1,42 resulting in the activation of several metabolic and detoxification pathways (Figure 1). Whereas the AHR-mediated transcription is modulated by nuclear coactivators and corepressors, AHR signaling is abrogated through a negative feedback mechanism, in which the AHR/ARNT heterodimer stimulates the expression of AHR repressor (AHRR) that competes with ARNT for binding to AHR.43 Alternatively, AHR may undergo nuclear export, ubiquitination and subsequent degradation by the 26S proteasome.44
Figure 1.
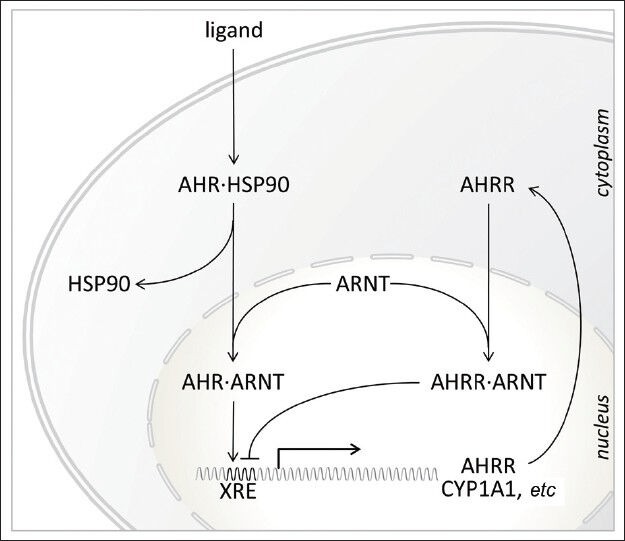
Schematic representation of ligand-activated AHR signaling. In the unliganded form, AHR resides in the cytoplasm as a complex with several chaperone proteins including heat shock protein 90 (HSP90). Ligand binding induces a conformational change in the AHR, revealing a nuclear localization signal that targets the AHR for nuclear translocation. In the nucleus, the AHR dimerizes with ARNT and interacts with the xenobiotic response element (XRE) in regulatory regions of specific target genes including AHRR and CYP1A1. In turn, AHRR acts as a repressor by competing for the dimerization with ARNT or directly at the XRE. AHR: aryl hydrocarbon receptor; AHRR: AHR repressor; ARNT, AHR nuclear translocator
EFFECTS OF DIOXIN EXPOSURE ON MALE REPRODUCTIVE HEALTH
Data on the effects of exposure to dioxins and dioxin-like compounds on male reproductive health have mainly been obtained from a few large-scale accidental human exposures including the one in Seveso, Italy, in a herbicide production plant in 1976,45 contaminated rice oil consumption in Japan (Yusho incident) in 196846 and in Taiwan region (Yucheng incident) in 197947 as well as US Air Force veterans who handled Agent Orange, a mixture of herbicides contaminated with TCDD, that was sprayed during operation Ranch Hand in Vietnam between 1962 and 1972.48
Boys that were exposed to TCDD in Seveso during infancy or puberty exhibited permanently reduced estradiol and increased follicle-stimulating hormone (FSH) levels,49 whereas boys born to Yucheng mothers showed decreased serum testosterone and increased serum estradiol and FSH at the age of puberty.50 An inverse relationship between dioxins and testosterone was also observed in Ranch Hand veterans51 and in men from the general population in Belgium.52 Results from the latter study further suggested that decreased testosterone levels may interfere with the secretory function of the prostate and seminal vesicles without affecting spermatogenesis. Exposure data from general populations are sparse, but in a study on Greenlandic Inuit and three European populations, inconsistent relationships were seen with gonadotropin, sex hormone binding globulin, estradiol and testosterone levels, where positive associations of PCB 153 were seen with sex hormone binding globulin and luteinizing hormone in some but not all groups.53
Widespread expression of AHR in the human testis may explain why dioxins and dioxin-like chemicals interfere with spermatogenesis and male fertility.54 Studies on reproductive function in sons of the exposed mothers from Seveso have shown that in utero and lactational exposure to relatively low dioxin doses can permanently reduce sperm quality, manifested as reductions in sperm concentration, total count, progressive motility and total motile count.45 Increased abnormal sperm morphology and decreased motility were also observed in a small cohort of young men born to Yucheng victims compared to controls, whereas semen volume and sperm count were not affected.55 Negative correlations between dioxin-like compounds and sperm quality have been supported by several relatively small studies,56,57,58 although a larger study that included 798 European men that were exposed to much lower levels did not confirm these associations.53 A recent study on 135 men from Seveso, who were exposed to TCDD during infancy, showed reduced sperm concentration and motility, whereas it had the opposite effect in men exposed during puberty and no effect in an older group with a mean age at exposure of 21.5 years.49 This indicates that exposure during the fetal or early life period is the most sensitive window of exposure. Indeed, studies on Agent Orange veterans, who were exposed during adult life, have also not shown associations between serum TCDD concentrations and sperm parameters.59 Reduced androgen levels during the period when Sertoli cells are most dependent on androgens could explain the permanently decreased sperm counts in adults who were exposed to TCDD before puberty.
The proportions of male births has slightly, but significantly, decreased in industrialized countries.60,61 Additionally, sharper changes in sex ratios have been observed in regions with demonstrated exposure to EDCs. For example, lower proportions of male offspring have been observed after paternal exposure to dioxin-like compounds in Seveso62 and Japan63 as well as in Russian pesticide producers.64 In the Yucheng cohort, birth sex ratio was not affected according to one study,65 whereas in another study exposed men had a lower proportion of male offspring than unexposed men when exposed before 20 years of age.66 Birth sex ratio was also not affected in US veterans who were exposed during adulthood.67
Interestingly, Taylor et al.68 reported that the proportion of male births was increased in women with higher estrogenic PCB levels, whereas it was decreased in women with higher levels of antiestrogenic PCBs.
In short, from these studies it is apparent that although exposure to dioxins or dioxin-like compounds may have a suppressive effect on testosterone levels throughout life, it only seems to affect male reproduction when exposed during reproductive development, i.e., either in utero or in the period up to puberty. Interestingly, increased PCB concentrations have been shown in mothers of men with testicular cancer but not in the men themselves.69
Crosstalk mechanisms between aryl hydrocarbon receptor and AR signaling pathways
Dioxins and dioxin-like compounds exert their biological and toxicological effects by activation of the AHR signaling pathway. The importance of the AHR signaling pathway in dioxin toxicity was independently demonstrated by three groups who developed Ahr-null mice that were highly resistant to diverse manifestations of dioxin toxicity.70,71,72 Similarly, mice that express low levels of ARNT from an engineered hypomorphic Arnt allele are highly resistant to hepatic toxicity of TCDD.73 But actions of the direct target genes of AHR alone do not fully explain its toxicological and physiological effects and it has become clear that AHR exhibits additional regulatory functions by modulating the activity of other signaling pathways. Proposed crosstalk occurs via mechanisms including competition for cofactors, protein-protein interaction, competition for DNA binding and proteasomal degradation. Crosstalk with the AHR signaling pathway has been demonstrated for estrogen receptor (ER) α,74 ER β75, hypoxia-inducible factor 1α,76 thyroid hormone receptor/retinoblastoma-interacting protein 230,77 nuclear factor erythroid 2-related factor 2,78 specificity protein 179 and nulcear factor-kB.80
AHR signaling may interfere with the male reproductive system through several mechanisms, for example by directly affecting steroid hormone levels via induction of CYP1A1 and CYP1B1, which are representative phase I drug metabolizing enzymes that catalyze the conversion of steroid hormones.81
Activation of AHR may also alter the transcriptional activity of steroid hormone receptors. TCDD has been shown to inhibit testosterone-dependent transcriptional activity and testosterone-regulated prostate specific antigen expression82 and to block androgen-dependent proliferation of prostate cancer cells.83 These in vitro findings are supported by a prospective cohort study of Vietnam veterans where an inverse relationship between TCDD body burden and risk of benign prostate hyperplasia was observed.51
The AHR has been shown to directly bind to a large number of coactivators and other nuclear proteins, including p300, cyclic AMP response element-binding (CREB)-binding protein, steroid receptor coactivators 1/2 and receptor-interacting protein 140.84,85 Many of these also interact with other nuclear receptors such as ER and the androgen receptor (AR), and as a result AHR and AR may compete with each other to recruit shared cofactors such as steroid receptor coactivators 1 and p300.86,87 Similar mechanisms have indeed been shown to account for the interaction between AHR and ER.88,89 It has been suggested that competition for the shared coactivator nuclear receptor coactivator 4, also known as AR associated protein 70, provides the basis for the bilateral transcriptional interference and that AHR modulation of AR activity is differentially altered by the level of four and a half LIM domain 2 protein and the amount of AHR present in the cell.90 Furthermore, PAHs have been shown to stimulate c-jun and c-fos expression in prostate cancer LNCaP cells.91 Since activator protein-1, a heterodimer of c-jun and c-fos, is known to inhibit binding of AR to androgen responsive elements by protein-protein interaction with AR, this suggests the involvement of AHR-induced activator protein-1 in the antiandrogenic effects of PAHs. Recently, Bjork and Giwercman reported that the suppressive effect of TCDD on AR activity depends on the polymorphic glutamine repeat in the transactivating domain of AR,92 lending further support that crosstalk between AR and AHR signaling is mediated at the level of cofactor binding.
Conversely, testosterone has been reported to repress TCDD-induced transcription of AHR-regulated CYP1A1 gene and CYP1A1 enzymatic activity in LNCaP cells.82,93 Furthermore, 5α-dihydrotestosterone was shown to suppress transcription of AHR-regulated genes by facilitating complex formation between AR and AHR, which results in reduced transcriptional activity.94
Crosstalk between AHR and AR signaling pathways could also occur by direct competition for DNA binding sites in the promoter of androgen-responsive genes, as has been shown for ER-regulated genes.95 TCDD treatment blocked ER binding to estrogen responsive elements and, conversely, estradiol blocked TCDD mediated CYP1A1 enzymatic activity by decreasing AHR binding to dioxin responsive elements in breast cancer cells.
Finally, AR signaling can be abrogated by the ability of the AHR to assemble an ubiquitin ligase complex, which subsequently promotes proteasomal degradation of the AR protein.96,97
Genetic polymorphisms
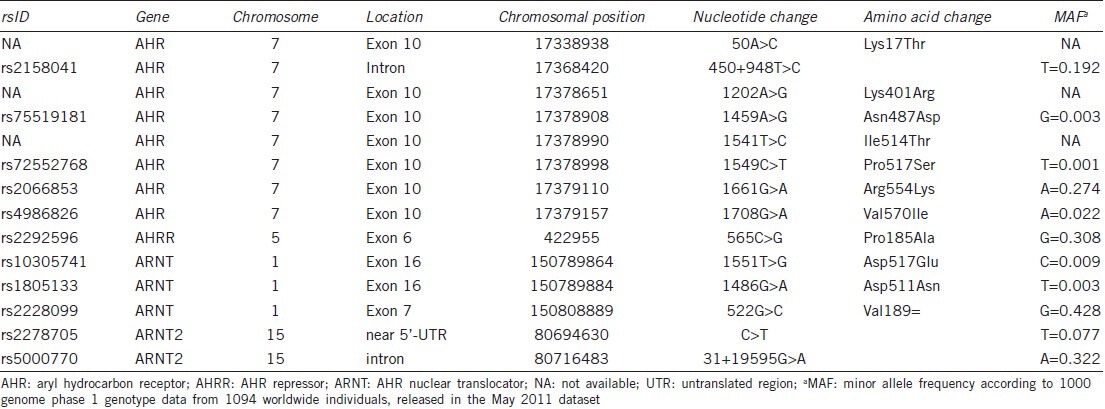
Polymorphisms in the AHR gene, enzymes that are transcriptionally regulated by AHR, or other genes involved in the AHR signaling pathway, may not only cause variations in the individual susceptibility to dioxin-like compounds, but may also affect the cross talk between AHR signaling and other signaling pathways as described above. As such, these polymorphisms may determine to what extent dioxins and dioxin-like compounds disrupt for example androgen signaling. Details of the polymorphisms discussed in this paper are provided in Table 1.
Table 1.
Details of polymorphisms discussed in the text according to dbSNP build 137
Genetic variations in the rodent AHR have been shown to dramatically alter ligand binding and transactivation by the receptor. For example, a single nucleotide change at codon 375 in the ligand binding domain of the murine AHR reduces the binding affinity for TCDD approximately 10-fold in the resistant DBA/2 strain as compared to the sensitive C57BL/6J strain.11,98 Correspondingly, the latter shows higher CYP1A1 induction and a greater sensitivity to TCDD.99 Due to a deletion in the transactivation domain of the rat AHR, the Han/Wistar rat strain is a 1000-fold more resistant to TCDD than the sensitive Long-Evans rat strain.12,100 The most dioxin-sensitive species is the Guinea pig, while the hamster, which has a modified transactivation domain similar to the resistant Han/Wistar rat, tolerates a 1000-fold higher dose.101,102 Interestingly, although humans are considered to be relatively insensitive to dioxins, the human AHR is highly homologous to the guinea pig AHR.103 Besides important implications for testing of pollutants in animal models, these inter- and intraspecies differences indicate that genetic polymorphisms in the AHR structure can have profound effects on the individual sensitivity to polycyclic and halogenated aromatic hydrocarbons.
In humans, genetic polymorphisms have been identified in the coding regions of the genes encoding AHR, ARNT and AHRR. The first identified and most widely studied single-nucleotide polymorphism (SNP) in the human AHR gene is a G > A substitution in exon 10, which causes an arginine to lysine change at codon 554 (Arg554Lys) in the transactivating domain of the receptor.104 Its functional significance is currently unclear as both upregulation105 as well as loss106 of transactivational activity have been reported for the lysine variant. Conflicting associations of this SNP with human cancer risk exist, but in a recent systematic meta-analysis Luo et al.107 concluded that this SNP does not contribute to the development of cancer.
The Arg554Lys SNP is in linkage disequilibrium with two other non-synonymous SNPs in exon 10 (Pro517Ser and Val570Ile), which are very rare except in African ancestry.105,106,108 Combinations of Lys554/Ile570 or Lys554/Ile570/Ser517 variant alleles are unable to drive the CYP1A1 gene expression in vitro.106 This could be beneficial to individuals who are carriers of these nonresponsive genotypes and who are exposed to AHR inducing chemicals. This is supported by the nearly total resistance to tumor formation in benzo[a]pyrene-exposed AHR knockout mice, also displaying loss of the ability to induce CYP1A1.109 A recent study confirmed that lower AHR, ARNT and CYP1B1 mRNA expression was associated with the homozygous variant Lys554 genotype of the AHR,110 but it remains to be seen whether humans who carry the variant codons at 570 and 517 will have a lower cancer risk as well.
Four additional human AHR variants (Lys17Thr, Lys401Arg, Asn487Asp and Ile514Thr) have been described.111 Reduced transcriptional activity was reported in the Lys401Arg and Asn487Asp variants, but so far the phenotypic consequences of these variants remain unknown.
The AHRR gene harbors a missense mutation leading to a Pro185Ala amino acid change in exon 6.112,113 Although the functional properties of this variant are unclear, it has been linked with endometriosis in women114 and infertility in men,115,116,117 possibly through a reduced negative feedback on dioxin-induced AHR signaling.118 Since this SNP influences CYP1A2 activation in vivo, with carriers of the Ala-genotype being the most inducible,119 the 185Ala is suggested to have a lower repressor activity towards the AHR. Recently, in a candidate association study on testicular cancer in a combined Swedish and Danish cohort, it was shown that the risk of disseminated TGCC was associated with four different SNPs that tag two haplotypes in the AHRR, whereas no association was found with SNPs in the AHR.120
In the ARNT gene (also known as ARNT1 and HIF1β), a silent mutation at codon 189 in exon 7 has been identified.121 The functional significance of this SNP is unknown; in smokers it did not affect CYP1A1 activity.122 Two other variants, Asp511Asn and Asp517Glu, have also been identified in the ARNT gene, but since both are located in exon 16, which does not contain a known functional domain, the significance of these SNPs has not been determined to date. Taken together, known variations in ARNT do probably not explain susceptibility to dioxins.123
In ARNT2 on the other hand, which is a close structural homologue of ARNT38 and expressed in parallel with ARNT in many tissues,124 a significant association was observed between two SNPs (rs2278705 and rs5000770) and having either cryptorchidism, hypospadias or both in Japanese boys, whereas, rs5000770 was linked to at least one genital malformation in Italian men.125 Studies in populations that have been exposed to significant dioxin levels may be needed to further confirm the possible role of ARNT or ANRT2 variants in male genital development.
One could argue that the human AHR is not likely to be polymorphic with respect to susceptibility for two reasons: (i) the human AHR already harbors the mutation that in the DBA/2-mouse reduces its affinity or CYP1A1 inducibility, which may not be overcome by additional mutations and (ii) the critical importance of the AHR during development may not allow additional deleterious mutations in this gene.126 However, a recent genetic association study in a Chinese cohort of 580 idiopathic infertile men and 580 fertile controls observed that men homozygous for the AHR rs2158041 AA genotype had lower sperm counts than carriers of the GG genotype.127 Interestingly, the same polymorphism also associated with risk of lung cancer in a similarly-sized Chinese study.128 The molecular mechanism underlying these associations is currently not clear since the SNP is intronic and therefore not expected to have a functional consequence. However, intronic SNPs can affect transcription,129 produce alternative splice sites130 or be in linkage disequilibrium with other, causal genes.
Gene-environment interactions
The concept of gene-environment interaction means that some people carry genetic factors that confer susceptibility or resistance to a certain disorder in a particular environment. Unfortunately, only few studies have been performed aiming at identifying gene-environment interactions with respect to male reproductive health. Animal studies have shown that daily sperm production is affected less by TCDD exposure in utero131 as well as during adulthood132 in resistant Han/Wistar rats who carry a mutated transactivation domain of the AHR as compared to Long-Evans rats carrying the wildtype allele.
In humans, studies on gene-environment interactions related to environmental exposure and male reproductive health are still limited, and have recently been reviewed by Axelsson et al.133 For example, a polymorphism in CYP1A1 and hydroxysteroid 17b-dehydrogenase 4 modifies the association between exposure levels to different PCB congeners and the risk of testicular cancer.134 Men with a shorter polymorphic trinucleotide CAG repeat length in AR have been observed to be more sensitive to the deleterious effects of exposure to PCB and p, p’-1,1-dichloro-2,2-bis (p-chlorophenyl) ethylene (DDE, a metabolite of the pesticide dichlorodiphenyltrichloroethane) on sperm DNA fragmentation and total sperm counts, respectively.135 Another study reported increased sperm DNA fragmentation in men with a variant of the detoxifying enzyme glutathione-S-transferase M1 when exposed to polycyclic aromatic hydrocarbon metabolites found in air pollution.136
Is there a case to be made for gene-environment interactions with respect to exposure to dioxins and dioxin-like compounds? On the one hand, exposure to these chemicals clearly has antiandrogenic effects on male reproductive health, especially when the exposure occurs during sensitive periods before adulthood, affecting reproductive hormone levels and ultimately sperm quality. On the other hand, polymorphisms in genes involved in AHR signaling pathway have been identified in humans, and in a limited number of studies these have been associated with male reproductive functions. Whether the antiandrogenic effects of dioxin-induced AHR signaling are mediated via cross talk with the AR, remains to be studied in more detail.
Although animals show large differences in sensitivity to dioxins due to these polymorphisms, the functional effects are less obvious in humans. One of the reasons for this discrepancy is that most genetic polymorphisms in AHR, ARNT or AHRR have first been identified by massive high-throughput screening, without known phenotypes, whereas it was the opposite in animal models, i.e. genotypic variation was studied as a consequence of differences in sensitivity in the different strains. Genetic analyses of these genes in populations that are exposed to wide range of exposure levels may identify yet unknown polymorphisms that to a larger extend explain variation in susceptibility to EDCs.
CONCLUDING REMARKS
Virtually all human diseases result from the interaction between genetic susceptibility factors and modifiable environmental factors. Many environmental pollutants exert there biological effects through activation of the AHR signaling cascade and polymorphisms in the genes involved in this pathway may affect an individual's response to these pollutants. It may not be likely that polymorphisms in the human AHR gene affect receptor function, but there is evidence to suggest that polymorphisms in AHRR may indeed affect an individual's susceptibility to dioxin-related reproductive health effects. Whether these anti-androgenic effects are indeed mediated through cross talk between AHR and AR signaling, remains to be scrutinized. Whereas the mechanisms underlying the cross talk between ER and AHR signaling have been well-described, only a few studies have addressed similar mechanisms between AR and AHR.
When studying such complex mechanisms as gene-environment interactions, even relatively large-scale multicenter studies have a relatively low statistical power. Therefore, efforts should be made to establish international consortia merging different cohorts from which biological material for genetic as well as exposure analyses is available. To facilitate such collaboration, international standards regarding type of biological samples and questionnaire information to be collected as well should be developed. Furthermore, taking into consideration the limited availability of sufficiently sized study cohorts, efforts should be made to develop more efficient study designs and statistical models for studying gene-environment interactions.137,138
QUESTIONS FROM THE PANEL
Q1: What can we learn from animal studies?
A1: Genetic polymorphisms in animals have convincingly been shown to affect susceptibility to dioxin-like compounds. However, given the fact that AHR receptor signaling is crucially important during development, the human AHR which already harbors a polymorphism that in the mouse reduces its activity may not allow further compromising genetic modifications.
Q2: Include gene-environment interaction studies with lifestyle factors as the “environment”.
A2: Smoking, which globally is an abundant lifestyle factor, has in a laboratory study been shown to affect men and women differently, so that women experienced greater abstinence induced anger than men, which could be an important factor for understanding and treating nicotine addiction in women.139 In a study on more than 15 000 adolescents, these were shown to smoke more cigarettes and consume more alcohol when attending schools with elevated rates of tobacco and alcohol use. More important, an individual's susceptibility to school-level patterns of smoking or drinking is conditional on the number of short alleles he or she has in 5HTTLPR (serotonin-transporter-linked polymorphic region), which is a polymorphic region in SLC6A4, the gene that codes for the serotonin transporter. Overall, the findings demonstrate the utility of the differential susceptibility framework by suggesting that health behaviors reflect interactions between genetic factors and the prevalence of these behaviors in a person's context.140
Q3: What is known about the genetic background in the susceptibility to certain EDCs in animals and humans? Are there indications of more or less susceptible subpopulations?
A3: Genetic variations in AHR in different rodent strains, as well as between different species, have indeed been shown to dramatically alter ligand binding and transactivation by the receptor, as detailed in the text. For example, the DBA/2 mouse strain is more resistant than the sensitive C57BL/6J strain,11,98,99 and the Han/Wistar rat strain is a 1000-fold more resistant to TCDD than the sensitive Long-Evans rat strain.12,100 The most dioxin-sensitive species is the guinea pig, while the hamster tolerates a 1000-fold higher dose.101,102
Studies in humans have shown that serum level of CB-153, a marker for PCB exposure, is positively associated with DNA fragmentation. Interestingly, this was only seen in Caucasian but not in Inuit men.141,142 Whether this difference is solely due to difference in genetic background, or also related to other environmental exposures or lifestyle factors, remains to be seen.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richiardi L, Bellocco R, Adami HO, Torrang A, Barlow L, et al. Testicular cancer incidence in eight northern European countries: secular and recent trends. Cancer Epidemiol Biomarkers Prev. 2004;13:2157–66. [PubMed] [Google Scholar]
- 3.Matlai P, Beral V. Trends in congenital malformations of external genitalia. Lancet. 1985;1:108. doi: 10.1016/s0140-6736(85)91999-3. [DOI] [PubMed] [Google Scholar]
- 4.Hemminki K, Li X. Cancer risks in Nordic immigrants and their offspring in Sweden. Eur J Cancer. 2002;38:2428–34. doi: 10.1016/s0959-8049(02)00496-3. [DOI] [PubMed] [Google Scholar]
- 5.McGlynn KA, Graubard BI, Klebanoff MA, Longnecker MP. Risk factors for cryptorchism among populations at differing risks of testicular cancer. Int J Epidemiol. 2006;35:787–95. doi: 10.1093/ije/dyl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giwercman YL, Kleist KE, Giwercman A, Giwercman C, Toft G, et al. Remarkably low incidence of hypospadias in Greenland despite high exposure to endocrine disrupters;possible protective effect of androgen receptor genotype. Pharmacogenet Genomics. 2006;16:375–7. doi: 10.1097/01.fpc.0000199497.01101.93. [DOI] [PubMed] [Google Scholar]
- 7.Chia VM, Quraishi SM, Devesa SS, Purdue MP, Cook MB, et al. International trends in the incidence of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev. 2010;19:1151–9. doi: 10.1158/1055-9965.EPI-10-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah MN, Devesa SS, Zhu K, McGlynn KA. Trends in testicular germ cell tumours by ethnic group in the United States. Int J Androl. 2007;30:206–13. doi: 10.1111/j.1365-2605.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 9.Kheirandish P, Chinegwundoh F. Ethnic differences in prostate cancer. Br J Cancer. 2011;105:481–5. doi: 10.1038/bjc.2011.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giwercman C, Giwercman A, Pedersen HS, Toft G, Lundin K, et al. Polymorphisms in genes regulating androgen activity among prostate cancer low-risk Inuit men and high-risk Scandinavians. Int. J. Androl. 2008;31:25–30. doi: 10.1111/j.1365-2605.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- 11.Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol Pharmacol. 1994;46:915–21. [PubMed] [Google Scholar]
- 12.Pohjanvirta R, Unkila M, Tuomisto J. Comparative acute lethality of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 1,2,3,7,8-pentachlorodibenzo-p-dioxin and 1,2,3,4,7,8-hexachlorodibenzo-p-dioxin in the most TCDD-susceptible and the most TCDD-resistant rat strain. Pharmacol Toxicol. 1993;73:52–6. doi: 10.1111/j.1600-0773.1993.tb01958.x. [DOI] [PubMed] [Google Scholar]
- 13.Franks PW. Genexenvironment interactions in type 2 diabetes. Curr Diab Rep. 2011;11:552–61. doi: 10.1007/s11892-011-0224-9. [DOI] [PubMed] [Google Scholar]
- 14.Ulrich CM, Kampman E, Bigler J, Schwartz SM, Chen C, et al. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction? Cancer Epidemiol Biomarkers Prev. 1999;8:659–68. [PubMed] [Google Scholar]
- 15.Talmud PJ. Gene-environment interaction and its impact on coronary heart disease risk. Nutr Metab Cardiovasc Dis. 2007;17:148–52. doi: 10.1016/j.numecd.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–46. [PubMed] [Google Scholar]
- 17.Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys. 1998;357:155–63. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 18.Sinal CJ, Bend JR. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol Pharmacol. 1997;52:590–9. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- 19.Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38:7594–600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- 20.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 21.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141:131–60. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez FJ, Fernandez-Salguero P, Lee SS, Pineau T, Ward JM. Xenobiotic receptor knockout mice. Toxicol Lett. 1995;82-83:117–21. doi: 10.1016/0378-4274(95)03548-6. [DOI] [PubMed] [Google Scholar]
- 24.Lahvis GP, Pyzalski RW, Glover E, Pitot HC, McElwee MK, et al. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol. 2005;67:714–20. doi: 10.1124/mol.104.008888. [DOI] [PubMed] [Google Scholar]
- 25.Lin TM, Ko K, Moore RW, Buchanan DL, Cooke PS, et al. Role of the aryl hydrocarbon receptor in the development of control and 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed male mice. J Toxicol Environ Health A. 2001;64:327–42. doi: 10.1080/152873901316981312. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–40. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–5. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 28.Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–48. doi: 10.1053/j.gastro.2011.04.007. 248 e1. [DOI] [PubMed] [Google Scholar]
- 29.Gasiewicz TA, Singh KP, Casado FL. The aryl hydrocarbon receptor has an important role in the regulation of hematopoiesis: implications for benzene-induced hematopoietic toxicity. Chem Biol Interact. 2010;184:246–51. doi: 10.1016/j.cbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baba T, Shima Y, Owaki A, Mimura J, Oshima M, et al. Disruption of aryl hydrocarbon receptor (AhR) induces regression of the seminal vesicle in aged male mice. Sex Dev. 2008;2:1–11. doi: 10.1159/000117714. [DOI] [PubMed] [Google Scholar]
- 31.Lin TM, Ko K, Moore RW, Simanainen U, Oberley TD, et al. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;68:479–87. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- 32.Fritz WA, Lin TM, Cardiff RD, Peterson RE. The aryl hydrocarbon receptor inhibits prostate carcinogenesis in TRAMP mice. Carcinogenesis. 2007;28:497–505. doi: 10.1093/carcin/bgl179. [DOI] [PubMed] [Google Scholar]
- 33.Moennikes O, Loeppen S, Buchmann A, Andersson P, Ittrich C, et al. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 2004;64:4707–10. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- 34.Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci U S A. 2002;99:9990–5. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukunaga BN, Probst MR, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem. 1995;270:29270–8. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 36.Ema M, Morita M, Ikawa S, Tanaka M, Matsuda Y, et al. Two new members of the murine Sim gene family are transcriptional repressors and show different expression patterns during mouse embryogenesis. Mol Cell Biol. 1996;16:5865–75. doi: 10.1128/mcb.16.10.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–9. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 38.Hirose K, Morita M, Ema M, Mimura J, Hamada H, et al. cDNA cloning and tissue-specific expression of a novel basic helix-loop-helix/PAS factor (Arnt2) with close sequence similarity to the aryl hydrocarbon receptor nuclear translocator (Arnt) Mol Cell Biol. 1996;16:1706–13. doi: 10.1128/mcb.16.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–8. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 40.Takahata S, Sogawa K, Kobayashi A, Ema M, Mimura J, et al. Transcriptionally active heterodimer formation of an Arnt-like PAS protein, Arnt3, with HIF-1a, HLF, and clock. Biochem Biophys Res Commun. 1998;248:789–94. doi: 10.1006/bbrc.1998.9012. [DOI] [PubMed] [Google Scholar]
- 41.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–5. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 42.McLane KE, Whitlock JP., Jr DNA sequence requirements for Ah receptor/Arnt recognition determined by in vitro transcription. Receptor. 1994;4:209–22. [PubMed] [Google Scholar]
- 43.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13:20–5. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prokipcak RD, Okey AB. Downregulation of the Ah receptor in mouse hepatoma cells treated in culture with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Can J Physiol Pharmacol. 1991;69:1204–10. doi: 10.1139/y91-176. [DOI] [PubMed] [Google Scholar]
- 45.Mocarelli P, Gerthoux PM, Needham LL, Patterson DG, Jr, Limonta G, et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119:713–8. doi: 10.1289/ehp.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuratsune M, Yoshimura T, Matsuzaka J, Yamaguchi A. Epidemiologic study on Yusho, a Poisoning Caused by Ingestion of Rice Oil Contaminated with a Commercial Brand of Polychlorinated Biphenyls. Environ Health Perspect. 1972;1:119–28. doi: 10.1289/ehp.7201119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang WY. PCBs in rice oil. Nature. 1982;296:192. doi: 10.1038/296192e0. [DOI] [PubMed] [Google Scholar]
- 48.Buckingham WA. Washington: U.S. Air Force Office of Air Force History; 1982. Operation Ranch Hand: The Air Force and Herbicides in Southeast Asia, 1961-1971; p. 253. [Google Scholar]
- 49.Mocarelli P, Gerthoux PM, Patterson DG, Jr, Milani S, Limonta G, et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116:70–7. doi: 10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu PC, Lai TJ, Guo NW, Lambert GH, Guo YL. Serum hormones in boys prenatally exposed to polychlorinated biphenyls and dibenzofurans. J Toxicol Environ Health A. 2005;68:1447–56. doi: 10.1080/15287390590967360. [DOI] [PubMed] [Google Scholar]
- 51.Gupta A, Ketchum N, Roehrborn CG, Schecter A, Aragaki CC, et al. Serum dioxin, testosterone, and subsequent risk of benign prostatic hyperplasia: a prospective cohort study of Air Force veterans. Environ Health Perspect. 2006;114:1649–54. doi: 10.1289/ehp.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhooge W, van Larebeke N, Koppen G, Nelen V, Schoeters G, et al. Serum dioxin-like activity is associated with reproductive parameters in young men from the general Flemish population. Environ Health Perspect. 2006;114:1670–6. doi: 10.1289/ehp.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toft G, Axmon A, Giwercman A, Thulstrup AM, Rignell-Hydbom A, et al. Fertility in four regions spanning large contrasts in serum levels of widespread persistent organochlorines: a cross-sectional study. Environ Health. 2005;4:26. doi: 10.1186/1476-069X-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schultz R, Suominen J, Varre T, Hakovirta H, Parvinen M, et al. Expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator messenger ribonucleic acids and proteins in rat and human testis. Endocrinology. 2003;144:767–76. doi: 10.1210/en.2002-220642. [DOI] [PubMed] [Google Scholar]
- 55.Guo YL, Hsu PC, Hsu CC, Lambert GH. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. Lancet. 2000;356:1240–1. doi: 10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- 56.Rozati R, Reddy PP, Reddanna P, Mujtaba R. Role of environmental estrogens in the deterioration of male factor fertility. Fertil Steril. 2002;78:1187–94. doi: 10.1016/s0015-0282(02)04389-3. [DOI] [PubMed] [Google Scholar]
- 57.Dallinga JW, Moonen EJ, Dumoulin JC, Evers JL, Geraedts JP, et al. Decreased human semen quality and organochlorine compounds in blood. Hum Reprod. 2002;17:1973–9. doi: 10.1093/humrep/17.8.1973. [DOI] [PubMed] [Google Scholar]
- 58.Hauser R, Altshul L, Chen Z, Ryan L, Overstreet J, et al. Environmental organochlorines and semen quality: results of a pilot study. Environ Health Perspect. 2002;110:229–33. doi: 10.1289/ehp.02110229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henriksen GL, Michalek JE. Serum dioxin, testosterone, and gonadotropins in veterans of Operation Ranch Hand. Epidemiology. 1996;7:454–5. [PubMed] [Google Scholar]
- 60.Moller H. Trends in sex-ratio, testicular cancer and male reproductive hazards: are they connected? APMIS. 1998;106:232. doi: 10.1111/j.1699-0463.1998.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 61.Davis DL, Webster P, Stainthorpe H, Chilton J, Jones L, et al. Declines in sex ratio at birth and fetal deaths in Japan, and in U.S. whites but not African Americans. Environ Health Perspect. 2007;115:941–6. doi: 10.1289/ehp.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mocarelli P, Gerthoux PM, Ferrari E, Patterson DG, Jr, Kieszak SM, et al. Paternal concentrations of dioxin and sex ratio of offspring. Lancet. 2000;355:1858–63. doi: 10.1016/S0140-6736(00)02290-X. [DOI] [PubMed] [Google Scholar]
- 63.Yorifuji T, Kashima S, Tokinobu A, Kato T, Tsuda T. Regional impact of exposure to a polychlorinated biphenyl and polychlorinated dibenzofuran mixture from contaminated rice oil on stillbirth rate and secondary sex ratio. Environ Int. 2013;59:12–5. doi: 10.1016/j.envint.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Ryan JJ, Amirova Z, Carrier G. Sex ratios of children of Russian pesticide producers exposed to dioxin. Environ Health Perspect. 2002;110:A699–701. doi: 10.1289/ehp.021100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rogan WJ, Gladen BC, Guo YL, Hsu CC. Sex ratio after exposure to dioxin-like chemicals in Taiwan. Lancet. 1999;353:206–7. doi: 10.1016/S0140-6736(05)77215-9. [DOI] [PubMed] [Google Scholar]
- 66.del Rio Gomez I, Marshall T, Tsai P, Shao YS, Guo YL. Number of boys born to men exposed to polychlorinated byphenyls. Lancet. 2002;360:143–4. doi: 10.1016/s0140-6736(02)09386-8. [DOI] [PubMed] [Google Scholar]
- 67.Schnorr TM, Lawson CC, Whelan EA, Dankovic DA, Deddens JA, et al. Spontaneous abortion, sex ratio, and paternal occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ Health Perspect. 2001;109:1127–32. doi: 10.1289/ehp.011091127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor KC, Jackson LW, Lynch CD, Kostyniak PJ, Buck Louis GM. Preconception maternal polychlorinated biphenyl concentrations and the secondary sex ratio. Environ Res. 2007;103:99–105. doi: 10.1016/j.envres.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Hardell L, van Bavel B, Lindstrom G, Carlberg M, Dreifaldt AC, et al. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ Health Perspect. 2003;111:930–4. doi: 10.1289/ehp.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–9. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 71.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–54. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 72.Lahvis GP, Bradfield CA. Ahr null alleles: distinctive or different? Biochem Pharmacol. 1998;56:781–7. doi: 10.1016/s0006-2952(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 73.Walisser JA, Bunger MK, Glover E, Harstad EB, Bradfield CA. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem. 2004;279:16326–31. doi: 10.1074/jbc.M400784200. [DOI] [PubMed] [Google Scholar]
- 74.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–16. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 75.Brunnberg S, Pettersson K, Rydin E, Matthews J, Hanberg A, et al. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc Natl Acad Sci U. S. A. 2003;100:6517–22. doi: 10.1073/pnas.1136688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J Biol Chem. 1999;274:12115–23. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- 77.Beischlag TV, Taylor RT, Rose DW, Yoon D, Chen Y, et al. Recruitment of thyroid hormone receptor/retinoblastoma-interacting protein 230 by the aryl hydrocarbon receptor nuclear translocator is required for the transcriptional response to both dioxin and hypoxia. J Biol Chem. 2004;279:54620–8. doi: 10.1074/jbc.M410456200. [DOI] [PubMed] [Google Scholar]
- 78.Wang L, He X, Szklarz GD, Bi Y, Rojanasakul Y, et al. The aryl hydrocarbon receptor interacts with nuclear factor erythroid 2-related factor 2 to mediate induction of NAD (P) H:quinoneoxidoreductase 1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Arch Biochem Biophys. 2013;537:31–8. doi: 10.1016/j.abb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi A, Sogawa K, Fujii-Kuriyama Y. Cooperative interaction between AhR. Arnt and Sp1 for the drug-inducible expression of CYP1A1 gene. J Biol Chem. 1996;271:12310–6. doi: 10.1074/jbc.271.21.12310. [DOI] [PubMed] [Google Scholar]
- 80.Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274:510–5. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 81.Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013;368 doi: 10.1098/rstb.2012.0431. 20120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, et al. Cross-talk between 2,3,7,8-tetrachlorodibenzo-p-dioxin and testosterone signal transduction pathways in LNCaP prostate cancer cells. Biochem Biophys Res Commun. 1999;256:462–8. doi: 10.1006/bbrc.1999.0367. [DOI] [PubMed] [Google Scholar]
- 83.Barnes-Ellerbe S, Knudsen KE, Puga A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin blocks androgen-dependent cell proliferation of LNCaP cells through modulation of pRB phosphorylation. Mol Pharmacol. 2004;66:502–11. doi: 10.1124/mol.104.000356. [DOI] [PubMed] [Google Scholar]
- 84.Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–50. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys. 2005;433:379–86. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 86.Kumar MB, Perdew GH. Nuclear receptor coactivator SRC-1 interacts with the Q-rich subdomain of the AhR and modulates its transactivation potential. Gene Expr. 1999;8:273–86. [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi A, Numayama-Tsuruta K, Sogawa K, Fujii-Kuriyama Y. CBP/p300 functions as a possible transcriptional coactivator of Ah receptor nuclear translocator (Arnt) J Biochem. 1997;122:703–10. doi: 10.1093/oxfordjournals.jbchem.a021812. [DOI] [PubMed] [Google Scholar]
- 88.Matthews J, Gustafsson JA. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nuclear receptor signaling. 2006;4:e016. doi: 10.1621/nrs.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohtake F, Fujii-Kuriyama Y, Kawajiri K, Kato S. Cross-talk of dioxin and estrogen receptor signals through the ubiquitin system. J Steroid Biochem Mol Biol. 2011;127:102–7. doi: 10.1016/j.jsbmb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 90.Kollara A, Brown TJ. Four and a half LIM domain 2 alters the impact of aryl hydrocarbon receptor on androgen receptor transcriptional activity. J Steroid Biochem Mol Biol. 2010;118:51–8. doi: 10.1016/j.jsbmb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 91.Kizu R, Okamura K, Toriba A, Kakishima H, Mizokami A, et al. A role of aryl hydrocarbon receptor in the antiandrogenic effects of polycyclic aromatic hydrocarbons in LNCaP human prostate carcinoma cells. Arch Toxicol. 2003;77:335–43. doi: 10.1007/s00204-003-0454-y. [DOI] [PubMed] [Google Scholar]
- 92.Bjork C, Giwercman YL. Androgen receptor CAG repeat length modifies the effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on receptor activity in human prostate cells. Reprod Toxicol. 2013;35:144–9. doi: 10.1016/j.reprotox.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 93.Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, et al. Comparative effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on MCF-7, RL95-2, and LNCaP cells: role of target steroid hormones in cellular responsiveness to CYP1A1 induction. Mol Cell Biol Res Commun. 2000;4:174–80. doi: 10.1006/mcbr.2001.0275. [DOI] [PubMed] [Google Scholar]
- 94.Sanada N, Gotoh Y, Shimazawa R, Klinge CM, Kizu R. Repression of activated aryl hydrocarbon receptor-induced transcriptional activation by 5alpha-dihydrotestosterone in human prostate cancer LNCaP and human breast cancer T47D cells. J Pharmacol Sci. 2009;109:380–7. doi: 10.1254/jphs.08328fp. [DOI] [PubMed] [Google Scholar]
- 95.Kharat I, Saatcioglu F. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin are mediated by direct transcriptional interference with the liganded estrogen receptor. Cross-talk between aryl hydrocarbon- and estrogen-mediated signaling. J Biol Chem. 1996;271:10533–7. doi: 10.1074/jbc.271.18.10533. [DOI] [PubMed] [Google Scholar]
- 96.Ohtake F, Baba A, Fujii-Kuriyama Y, Kato S. Intrinsic AhR function underlies cross-talk of dioxins with sex hormone signalings. Biochem Biophys Res Commun. 2008;370:541–6. doi: 10.1016/j.bbrc.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 97.Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–6. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- 98.Chang C, Smith DR, Prasad VS, Sidman CL, Nebert DW, et al. Ten nucleotide differences, five of which cause amino acid changes, are associated with the Ah receptor locus polymorphism of C57BL/6 and DBA/2 mice. Pharmacogenetics. 1993;3:312–21. doi: 10.1097/00008571-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 99.Okey AB, Vella LM, Harper PA. Detection and characterization of a low affinity form of cytosolic Ah receptor in livers of mice nonresponsive to induction of cytochrome P1-450 by 3-methylcholanthrene. Mol Pharmacol. 1989;35:823–30. [PubMed] [Google Scholar]
- 100.Pohjanvirta R, Wong JM, Li W, Harper PA, Tuomisto J, et al. Point mutation in intron sequence causes altered carboxyl-terminal structure in the aryl hydrocarbon receptor of the most 2,3,7,8-tetrachlorodibenzo-p-dioxin-resistant rat strain. Mol Pharmacol. 1998;54:86–93. doi: 10.1124/mol.54.1.86. [DOI] [PubMed] [Google Scholar]
- 101.Olson JR, Gasiewicz TA, Neal RA. Tissue distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the Golden Syrian hamster. Toxicol Appl Pharmacol. 1980;56:78–85. doi: 10.1016/0041-008x(80)90132-5. [DOI] [PubMed] [Google Scholar]
- 102.Henck JM, New MA, Kociba RJ, Rao KS. 2,3,7,8-tetrachlorodibenzo-p-dioxin: acute oral toxicity in hamsters. Toxicol Appl Pharmacol. 1981;59:405–7. doi: 10.1016/0041-008x(81)90212-x. [DOI] [PubMed] [Google Scholar]
- 103.Korkalainen M, Tuomisto J, Pohjanvirta R. The AH receptor of the most dioxin-sensitive species, guinea pig, is highly homologous to the human AH receptor. Biochem Biophys Res Commun. 2001;285:1121–9. doi: 10.1006/bbrc.2001.5317. [DOI] [PubMed] [Google Scholar]
- 104.Kawajiri K, Watanabe J, Eguchi H, Hayashi S. Genetic polymorphisms of drug-metabolizing enzymes and lung cancer susceptibility. Pharmacogenetics. 1995;5(Spec No):S70–3. doi: 10.1097/00008571-199512001-00004. [DOI] [PubMed] [Google Scholar]
- 105.Smart J, Daly AK. Variation in induced CYP1A1 levels: relationship to CYP1A1, Ah receptor and GSTM1 polymorphisms. Pharmacogenetics. 2000;10:11–24. doi: 10.1097/00008571-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 106.Wong JM, Okey AB, Harper PA. Human aryl hydrocarbon receptor polymorphisms that result in loss of CYP1A1 induction. Biochem Biophys Res Commun. 2001;288:990–6. doi: 10.1006/bbrc.2001.5861. [DOI] [PubMed] [Google Scholar]
- 107.Luo C, Zou P, Ji G, Gu A, Zhao P, et al. The aryl hydrocarbon receptor (AhR) 1661G>A polymorphism in human cancer: a meta-analysis. Gene. 2013;513:225–30. doi: 10.1016/j.gene.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 108.Daly AK, Fairbrother KS, Smart J. Recent advances in understanding the molecular basis of polymorphisms in genes encoding cytochrome P450 enzymes. Toxicol Lett. 1998;102-103:143–7. doi: 10.1016/s0378-4274(98)00299-9. [DOI] [PubMed] [Google Scholar]
- 109.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, et al. Benzo[a] pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2000;97:779–82. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Helmig S, Seelinger JU, Dohrel J, Schneider J. RNA expressions of AHR, ARNT and CYP1B1 are influenced by AHR Arg554Lys polymorphism. Mol Genet Metab. 2011;104:180–4. doi: 10.1016/j.ymgme.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 111.Koyano S, Saito Y, Fukushima-Uesaka H, Ishida S, Ozawa S, et al. Functional analysis of six human aryl hydrocarbon receptor variants in a Japanese population. Drug Metab Dispos. 2005;33:1254–60. doi: 10.1124/dmd.105.004655. [DOI] [PubMed] [Google Scholar]
- 112.Cauchi S, Stucker I, Cenee S, Kremers P, Beaune P, et al. Structure and polymorphisms of human aryl hydrocarbon receptor repressor (AhRR) gene in a French population: relationship with CYP1A1 inducibility and lung cancer. Pharmacogenetics. 2003;13:339–47. doi: 10.1097/01.fpc.0000054093.48725.79. [DOI] [PubMed] [Google Scholar]
- 113.Watanabe T, Imoto I, Kosugi Y, Fukuda Y, Mimura J, et al. Human arylhydrocarbon receptor repressor (AHRR) gene: genomic structure and analysis of polymorphism in endometriosis. J Hum Genet. 2001;46:342–6. doi: 10.1007/s100380170070. [DOI] [PubMed] [Google Scholar]
- 114.Tsuchiya M, Katoh T, Motoyama H, Sasaki H, Tsugane S, et al. Analysis of the AhR, ARNT, and AhRR gene polymorphisms: genetic contribution to endometriosis susceptibility and severity. Fertil Steril. 2005;84:454–8. doi: 10.1016/j.fertnstert.2005.01.130. [DOI] [PubMed] [Google Scholar]
- 115.Fujita H, Kosaki R, Yoshihashi H, Ogata T, Tomita M, et al. Characterization of the aryl hydrocarbon receptor repressor gene and association of its Pro185Ala polymorphism with micropenis. Teratology. 2002;65:10–8. doi: 10.1002/tera.1093. [DOI] [PubMed] [Google Scholar]
- 116.Merisalu A, Punab M, Altmae Sb, Haller K, Tiido T, et al. The contribution of genetic variations of aryl hydrocarbon receptor pathway genes to male factor infertility. Fertil Steril. 2007;88:854–9. doi: 10.1016/j.fertnstert.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 117.Soneda S, Fukami M, Fujimoto M, Hasegawa T, Koitabashi Y, et al. Association of micropenis with Pro185Ala polymorphism of the gene for aryl hydrocarbon receptor repressor involved in dioxin signaling. Endocr J. 2005;52:83–8. doi: 10.1507/endocrj.52.83. [DOI] [PubMed] [Google Scholar]
- 118.Watanabe M, Sueoka K, Sasagawa I, Nakabayashi A, Yoshimura Y, et al. Association of male infertility with Pro185Ala polymorphism in the aryl hydrocarbon receptor repressor gene: implication for the susceptibility to dioxins. Fertil Steril. 2004;82(Suppl 3):1067–71. doi: 10.1016/j.fertnstert.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 119.Hung WT, Lambert GH, Huang PW, Patterson DG, Jr, Guo YL. Genetic susceptibility to dioxin-like chemicals’ induction of cytochrome P4501A2 in the human adult linked to specific AhRR polymorphism. Chemosphere. 2013;90:2358–64. doi: 10.1016/j.chemosphere.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 120.Brokken LJ, Lundberg-Giwercman Y, Meyts ER, Eberhard J, Stahl O, et al. Association between polymorphisms in the aryl hydrocarbon receptor repressor gene and disseminated testicular germ cell cancer. Front Endocrinol (Lausanne) 2013;4:4. doi: 10.3389/fendo.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scheel J, Hussong R, Schrenk D, Schmitz HJ. Variability of the human aryl hydrocarbon receptor nuclear translocator (ARNT) gene. J Hum Genet. 2002;47:217–24. doi: 10.1007/s100380200028. [DOI] [PubMed] [Google Scholar]
- 122.Anttila S, Lei XD, Elovaara E, Karjalainen A, Sun W, et al. An uncommon phenotype of poor inducibility of CYP1A1 in human lung is not ascribable to polymorphisms in the AHR, ARNT, or CYP1A1 genes. Pharmacogenetics. 2000;10:741–51. doi: 10.1097/00008571-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 123.Korkalainen M, Tuomisto J, Pohjanvirta R. Identification of novel splice variants of ARNT and ARNT2 in the rat. Biochem Biophys Res Commun. 2003;303:1095–100. doi: 10.1016/s0006-291x(03)00489-3. [DOI] [PubMed] [Google Scholar]
- 124.Aitola MH, Pelto-Huikko MT. Expression of Arnt and Arnt2 mRNA in developing murine tissues. J Histochem Cytochem. 2003;51:41–54. doi: 10.1177/002215540305100106. [DOI] [PubMed] [Google Scholar]
- 125.Qin XY, Kojima Y, Mizuno K, Ueoka K, Massart F, et al. Association of variants in genes involved in environmental chemical metabolism and risk of cryptorchidism and hypospadias. J Hum Genet. 2012;57:434–41. doi: 10.1038/jhg.2012.48. [DOI] [PubMed] [Google Scholar]
- 126.Connor KT, Aylward LL. Human response to dioxin: aryl hydrocarbon receptor (AhR) molecular structure, function, and dose-response data for enzyme induction indicate an impaired human AhR. J Toxicol Environ Health B Crit Rev. 2006;9:147–71. doi: 10.1080/15287390500196487. [DOI] [PubMed] [Google Scholar]
- 127.Gu A, Ji G, Long Y, Zhou Y, Shi X, et al. Assessment of an association between an aryl hydrocarbon receptor gene (AHR) polymorphism and risk of male infertility. Toxicol Sci. 2011;122:415–21. doi: 10.1093/toxsci/kfr137. [DOI] [PubMed] [Google Scholar]
- 128.Chen D, Tian T, Wang H, Liu H, Hu Z, et al. Association of human aryl hydrocarbon receptor gene polymorphisms with risk of lung cancer among cigarette smokers in a Chinese population. Pharmacogenet Genomics. 2009;19:25–34. doi: 10.1097/FPC.0b013e328316d8d8. [DOI] [PubMed] [Google Scholar]
- 129.Hugo H, Cures A, Suraweera N, Drabsch Y, Purcell D, et al. Mutations in the MYB intron I regulatory sequence increase transcription in colon cancers. Genes Chromosomes Cancer. 2006;45:1143–54. doi: 10.1002/gcc.20378. [DOI] [PubMed] [Google Scholar]
- 130.Moyer RA, Wang D, Papp AC, Smith RM, Duque L, et al. Intronic polymorphisms affecting alternative splicing of human dopamine D2 receptor are associated with cocaine abuse. Neuropsychopharmacology. 2011;36:753–62. doi: 10.1038/npp.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simanainen U, Tuomisto JT, Pohjanvirta R, Syrjala P, Tuomisto J, et al. Postnatal development of resistance to short-term high-dose toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in TCDD-resistant and -semiresistant rats. Toxicol Appl Pharmacol. 2004;196:11–9. doi: 10.1016/j.taap.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 132.Simanainen U, Haavisto T, Tuomisto JT, Paranko J, Toppari J, et al. Pattern of male reproductive system effects after in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure in three differentially TCDD-sensitive rat lines. Toxicol Sci. 2004;80:101–8. doi: 10.1093/toxsci/kfh142. [DOI] [PubMed] [Google Scholar]
- 133.Axelsson J, Bonde JP, Giwercman YL, Rylander L, Giwercman A. Gene-environment interaction and male reproductive function. Asian J Androl. 2010;12:298–307. doi: 10.1038/aja.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chia VM, Li Y, Quraishi SM, Graubard BI, Figueroa JD, et al. Effect modification of endocrine disruptors and testicular germ cell tumour risk by hormone-metabolizing genes. Int J Androl. 2010;33:588–96. doi: 10.1111/j.1365-2605.2009.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giwercman A, Rylander L, Rignell-Hydbom A, Jonsson BA, Pedersen HS, et al. Androgen receptor gene CAG repeat length as a modifier of the association between persistent organohalogen pollutant exposure markers and semen characteristics. Pharmacogenet Genomics. 2007;17:391–401. doi: 10.1097/01.fpc.0000236329.26551.78. [DOI] [PubMed] [Google Scholar]
- 136.Rubes J, Rybar R, Prinosilova P, Veznik Z, Chvatalova I, et al. Genetic polymorphisms influence the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat Res. 2010;683:9–15. doi: 10.1016/j.mrfmmm.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 137.Gauderman WJ, Thomas DC, Murcray CE, Conti D, Li D, et al. Efficient genome-wide association testing of gene-environment interaction in case-parent trios. Am J Epidemiol. 2010;172:116–22. doi: 10.1093/aje/kwq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cornelis MC, Tchetgen EJ, Liang L, Qi L, Chatterjee N, et al. Gene-environment interactions in genome-wide association studies: a comparative study of tests applied to empirical studies of type 2 diabetes. Am J Epidemiol. 2012;175:191–202. doi: 10.1093/aje/kwr368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pang RD, Leventhal AM. Sex differences in negative affect and lapse behavior during acute tobacco abstinence: a laboratory study. Exp Clin Psychopharmacol. 2013;21:269–76. doi: 10.1037/a0033429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daw J, Shanahan M, Harris KM, Smolen A, Haberstick B, et al. Genetic sensitivity to peer behaviors: 5HTTLPR, smoking, and alcohol consumption. J Health Soc Behav. 2013;54:92–108. doi: 10.1177/0022146512468591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rignell-Hydbom A, Rylander L, Giwercman A, Jonsson BA, Lindh C, et al. Exposure to PCBs and p, p’- DDE and human sperm chromatin integrity. Environ Health Perspect. 2005;113:175–9. doi: 10.1289/ehp.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bonde JP, Toft G, Rylander L, Rignell-Hydbom A, Giwercman A, et al. Fertility and markers of male reproductive function in Inuit and European populations spanning large contrasts in blood levels of persistent organochlorines. Environ Health Perspect. 2008;116:269–77. doi: 10.1289/ehp.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]


