Abstract
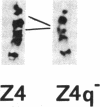
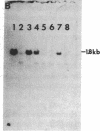
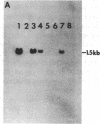
The CHO-AT3-2 Chinese hamster ovary cell line is functionally hemizygous for the adenine phosphoribosyltransferase (APRT; EC 2.4.2.7) locus. Class 1 APRT +/- heterozygotes, such as CHO-AT3-2, can be isolated at high spontaneous frequencies from wild-type CHO cell populations. Simon et al. [Simon, A. E., Taylor, M. W., Bradley, W. E. C. & Thompson, L. (1982) Mol. Cell. Biol. 2, 1126-1133] have proposed that a high-frequency event that inactivates one APRT allele might be responsible for both the spontaneous generation of class 1 APRT +/- heterozygotes and the high-frequency occurrence of APRT- mutants in class 2 APRT +/- heterozygote populations. This event appears to occur at only one of the two APRT alleles. To investigate the nature of this high-frequency event, and to determine the genetic basis for functional hemizygosity of the APRT locus in CHO-AT3-2 cells, we have mapped the APRT locus by using CHO-AT3-2-mouse somatic cell hybrids. Our data confirm that CHO-AT3-2 cells have a single functional APRT allele, which is located on the Z7 chromosome. Karyotypic analysis of CHO-AT3-2 revealed an interstitial deletion on the long arm of the Z4 chromosome, in the very region where the other APRT allele should be located. To determine whether the Z4q interstitial deletion had resulted in physical loss of the APRT gene, DNA from CHO-AT3-2-mouse cell hybrids that had either lost or retained the Z4q- chromosome was analyzed for the presence of CHO APRT coding sequences. Our data suggest that allele-specific high-frequency structural gene deletion events involving the long arm of chromosome Z4 are responsible for the spontaneous generation of functional hemizygosity at the APRT locus in CHO cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Carver J. H. Induction and expression of mutations at multiple drug-resistance marker loci in Chinese hamster ovary cells. Environ Mutagen. 1983;5(2):161–175. doi: 10.1002/em.2860050206. [DOI] [PubMed] [Google Scholar]
- Adair G. M., Carver J. H., Wandres D. L. Mutagenicity testing in mammalian cells. I. Derivation of a Chinese hamster ovary cell line heterozygous for the adenine phosphoribosyltransferase and thymidine kinase loci. Mutat Res. 1980 Sep;72(2):187–205. doi: 10.1016/0027-5107(80)90035-4. [DOI] [PubMed] [Google Scholar]
- Adair G. M., Stallings R. L., Friend K. K., Siciliano M. J. Gene mapping and linkage analysis in Chinese hamster: assignment of the genes for APRT, LDHA, IDH2, and GAA to chromosome 3. Somatic Cell Genet. 1983 Jul;9(4):477–487. doi: 10.1007/BF01543048. [DOI] [PubMed] [Google Scholar]
- Bradley W. E., Letovanec D. High-frequency nonrandom mutational event at the adenine phosphoribosyltransferase (aprt) locus of sib-selected CHO variants heterozygous for aprt. Somatic Cell Genet. 1982 Jan;8(1):51–66. doi: 10.1007/BF01538650. [DOI] [PubMed] [Google Scholar]
- Carver J. H., Adair G. M., Wandres D. L. Mutagenicity testing in mammalian cells. II. Validation of multiple drug-resistance markers having practical application for screening potential mutagens. Mutat Res. 1980 Sep;72(2):207–230. doi: 10.1016/0027-5107(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Chaleff D. T., Fink G. R. Genetic events associated with an insertion mutation in yeast. Cell. 1980 Aug;21(1):227–237. doi: 10.1016/0092-8674(80)90130-0. [DOI] [PubMed] [Google Scholar]
- Chasin L. A. Mutations affecting adenine phosphoribosyl transferase activity in Chinese hamster cells. Cell. 1974 May;2(1):37–41. doi: 10.1016/0092-8674(74)90006-3. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Erikson J., ar-Rushdi A., Drwinga H. L., Nowell P. C., Croce C. M. Transcriptional activation of the translocated c-myc oncogene in burkitt lymphoma. Proc Natl Acad Sci U S A. 1983 Feb;80(3):820–824. doi: 10.1073/pnas.80.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eves E. M., Farber R. A. Chromosome segregation is frequently associated with the expression of recessive mutations in mouse cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1768–1772. doi: 10.1073/pnas.78.3.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. Induction of thymidine kinase in enzyme-deficient Chinese hamster cells. Cell. 1982 Jun;29(2):483–492. doi: 10.1016/0092-8674(82)90165-9. [DOI] [PubMed] [Google Scholar]
- Hozier J., Sawyer J., Moore M., Howard B., Clive D. Cytogenetic analysis of the L5178Y/TK+/- leads to TK-/- mouse lymphoma mutagenesis assay system. Mutat Res. 1981 Nov;84(1):169–181. doi: 10.1016/0027-5107(81)90060-9. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983 Feb;32(2):311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- Kusano T., Long C., Green H. A new reduced human-mouse somatic cell hybrid containing the human gene for adenine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1971 Jan;68(1):82–86. doi: 10.1073/pnas.68.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Rubin G. M. The unstable wDZL mutation of Drosophila is caused by a 13 kilobase insertion that is imprecisely excised in phenotypic revertants. Cell. 1982 Sep;30(2):543–550. doi: 10.1016/0092-8674(82)90251-3. [DOI] [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Meuth M., Arrand J. E. Alterations of gene structure in ethyl methane sulfonate-induced mutants of mammalian cells. Mol Cell Biol. 1982 Nov;2(11):1459–1462. doi: 10.1128/mcb.2.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Genetic evidence for the inactivation of a human autosomal locus attached to an inactive X chromosome. Am J Hum Genet. 1982 Sep;34(5):811–817. [PMC free article] [PubMed] [Google Scholar]
- Morrow J. Gene inactivation as a mechanism for the generation of variability in somatic cells cultivated in vitro. Mutat Res. 1977 Sep;44(3):391–400. doi: 10.1016/0027-5107(77)90097-5. [DOI] [PubMed] [Google Scholar]
- Nairn R. S., Adair G. M., Humphrey R. M. DNA-mediated gene transfer in Chinese hamster ovary cells: clonal variation in transfer efficiency. Mol Gen Genet. 1982;187(3):384–390. doi: 10.1007/BF00332616. [DOI] [PubMed] [Google Scholar]
- Ray M., Mohandas T. Proposed banding nomenclature for the Chinese hamster chromosomes (Cricetulus griseus). Cytogenet Cell Genet. 1976;16(1-5):83–91. doi: 10.1159/000130559. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Axel R., Henderson A. S. Chromosome structure and DNA sequence alterations associated with mutation of transformed genes. J Mol Appl Genet. 1981;1(3):191–203. [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Siciliano M. J., Stallings R. L., Adair G. M., Humphrey R. M., Siciliano J. Provisional assignment of TPI, GPI, and PEPD to Chinese hamster autosomes 8 and 9: a cytogenetic basis for functional haploidy of an autosomal linkage group in CHO cells. Cytogenet Cell Genet. 1983;35(1):15–20. doi: 10.1159/000131830. [DOI] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W., Bradley W. E., Thompson L. H. Model involving gene inactivation in the generation of autosomal recessive mutants in mammalian cells in culture. Mol Cell Biol. 1982 Sep;2(9):1126–1133. doi: 10.1128/mcb.2.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. E., Taylor M. W. High-frequency mutation at the adenine phosphoribosyltransferase locus in Chinese hamster ovary cells due to deletion of the gene. Proc Natl Acad Sci U S A. 1983 Feb;80(3):810–814. doi: 10.1073/pnas.80.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings R. L., Siciliano M. J. Genetic polymorphisms and gene expression variations at enzyme loci in chinese hamster cell lines. Somatic Cell Genet. 1981 May;7(3):295–306. doi: 10.1007/BF01538855. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Tischfield J. A., Trill J. J., Lee Y. I., Coy K., Taylor M. W. Genetic instability at the adenine phosphoribosyltransferase locus in mouse L cells. Mol Cell Biol. 1982 Mar;2(3):250–257. doi: 10.1128/mcb.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]