Figure 4.
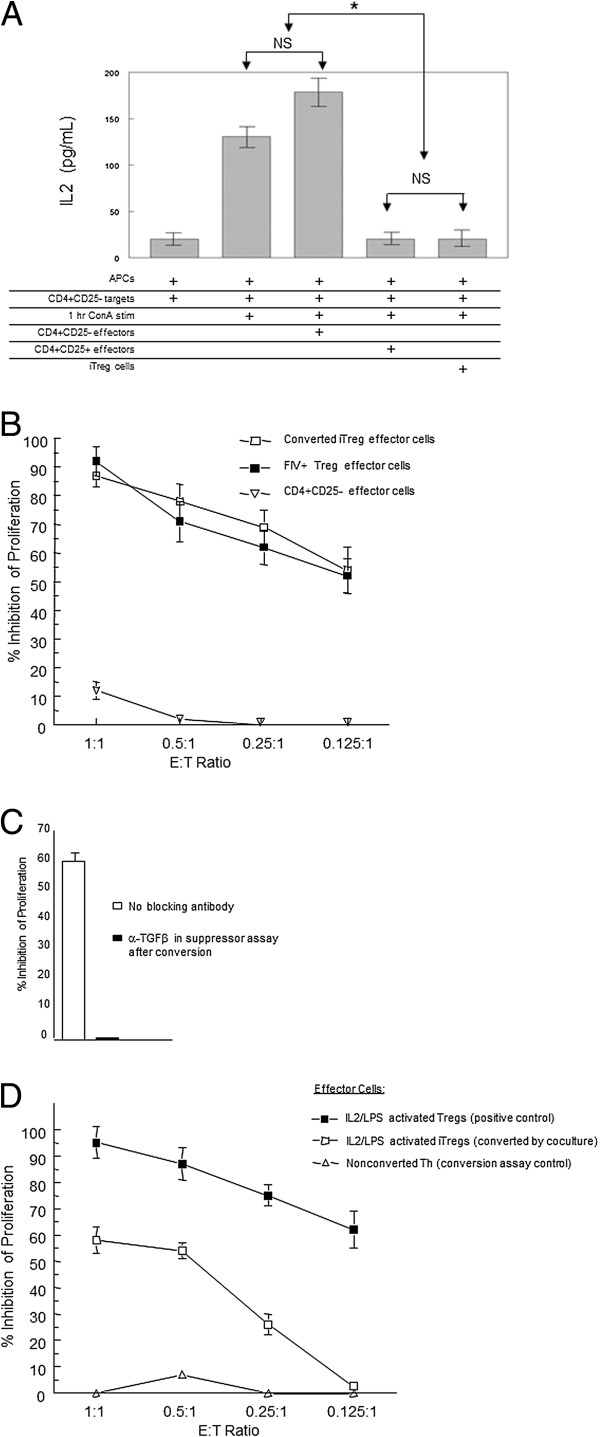
Activated feline CD4+CD25+T cells from FIV-infected cats convert CD4+CD25-Th cells into functional iTreg cells. CD4+CD25- cells were converted by co-culture with CD4+CD25+ T cells (outlined in Figure 2) and effector function analyzed. For suppression assays, CD4+CD25- (Th) and CD4-CD8- (APCs) from control cats were combined at a 1:1 ratio for use as targets and, where indicated, stimulated for 1 hour with ConA (5 ug/uL). A. Nonstimulated CD4+CD25- (negative control), LPS/IL2 activated CD4+CD25+ (positive control), or iTregs from conversion co-cultures with FIV+ Tregs were used as effectors. After 24 hours, IL2 was analyzed in the supernatent by ELISA in triplicate. Data shown is representative of two experiments. Error bars represent the SEM (*p < .05 Mann–Whitney). B. CD4+CD25- target cells were co-cultured with iTregs (open square), or freshly isolated CD4+CD25+ cells from an FIV+ cat (closed square), or CD4+CD25- cells (inverted triangle) at various E:T ratios for 72 hrs and pulsed with 3H-thymidine. Symbols are the mean ± SEM percent inhibition of proliferation of targets from three experiments. C. iTregs either untreated or pre-incubated with anti-TGFβ were used as effectors in a proliferation assay as described in B. Bars represent the mean ± SEM percent inhibition of proliferation of Th targets for three experiments. D. CD4+CD25- targets for the iTreg conversion assay were first cultured for 5 days with DiD-labeled CD4+CD25+ Tregs isolated from an FIV negative cat and activated by 5 day IL2/LPS treatment. Following co-culture, converted cells were isolated by FACS and used as effectors in a standard 72 hour 3H-thymidine proliferation assay with CD4+CD25- targets at various E:T ratios. Effector cell control for the assay was 5 day IL2/LPS activated CD4+CD25+ Tregs (closed square, positive control). Bars represent the mean ± SEM percent inhibition of proliferation of targets and represent three separate experiments.
