Abstract
Background
Prognostication in the early stage of traumatic coma is a common challenge in the neuro-intensive care unit. We report the unexpected recovery of functional milestones (i.e., consciousness, communication, and community reintegration) in a 19-year-old man who sustained a severe traumatic brain injury. The early magnetic resonance imaging (MRI) findings, at the time, suggested a poor prognosis.
Methods
During the first year of the patient’s recovery, MRI with diffusion tensor imaging (DTI) and T2*-weighted imaging was performed on day 8 (coma), day 44 (minimally conscious state), day 198 (post-traumatic confusional state), and day 366 (community reintegration). Mean apparent diffusion coefficient (ADC) and fractional anisotropy (FA) values in the corpus callosum, cerebral hemispheric white matter and thalamus were compared with clinical assessments using the Disability Rating Scale (DRS).
Results
Extensive diffusion restriction in the corpus callosum and bihemispheric white matter was observed on day 8, with ADC values in a range typically associated with neurotoxic injury (230 to 400 × 10−6 mm2/sec). T2*-weighted MRI revealed widespread hemorrhagic axonal injury in the cerebral hemispheres, corpus callosum, and brainstem. Despite the presence of severe axonal injury on early MRI, the patient regained the ability to communicate and perform activities of daily living independently at one year post-injury (DRS = 8).
Conclusions
MRI data should be interpreted with caution when prognosticating for patients in traumatic coma. Recovery of consciousness and community reintegration are possible even when extensive traumatic axonal injury is demonstrated by early MRI.
Keywords: Traumatic brain injury, Coma, Magnetic resonance imaging, Traumatic axonal injury, Diffusion-weighted imaging, Apparent diffusion coefficient, Diffusion tensor imaging
Introduction
Predicting recovery of consciousness and functional capacity in patients with severe traumatic brain injury (TBI) is a common challenge that confronts clinicians in civilian and military intensive care units [1]. Although the majority of patients survive [2], many suffer from severe disability [3] or remain in a vegetative [4] or minimally conscious state [5] after emergence from coma [6]. Recovery of meaningful neurological function has, however, been reported after traumatic coma [7–9], with up to 20% achieving a favorable outcome after a prolonged period of altered consciousness [6, 10–14]. Given the profound implications of neurological recovery for patients and their families, the development of clinical tools to predict outcomes in acute TBI has been the focus of several international studies over the past decade. These studies established the prognostic utility of demographic, physical examination, laboratory and computed tomography (CT) findings [15, 16], which formed the basis for the IMPACT [17] and MRC CRASH [18] outcome prediction models.
A major limitation of the IMPACT and MRC CRASH prognostic models is their reliance on CT imaging data, since recent studies have demonstrated that magnetic resonance imaging (MRI) provides better prognostic accuracy than does CT [7, 19, 20]. The advantage that MRI provides over CT is attributed to its increased sensitivity for identifying traumatic axonal injury (TAI) [20–23], which is the most difficult to detect [24] and neurologically devastating [25, 26] type of lesion in patients with non-penetrating head trauma. The MRI techniques that have shown greatest promise in improving the detection of TAI, and hence improving prognosis, are diffusion-weighted imaging (DWI) and T2*-weighted gradient-recalled echo (GRE) imaging, which are sensitive to non-hemorrhagic and hemorrhagic axonal injury, respectively [27]. Experimental [28] and clinical [29–31] studies utilizing DWI have shown that TAI is associated with early and persistent decreases in the apparent diffusion coefficient (ADC), similar to that observed in acute tissue infarction due to ischemia [32]. Furthermore, DWI studies in comatose cardiac arrest patients suggest that severe ADC reductions are predictive of poor long-term functional outcome [33–35]. These observations have led many to speculate that severe ADC reduction over large cerebral territories may also be predictive of poor outcome in comatose TBI patients. Similarly, T2*-weighted imaging has been shown to provide superior detection of hemorrhagic TAI compared to T2-weighted imaging and CT [36, 37].
In this report, we describe the unexpected extent of functional recovery demonstrated by a patient with traumatic coma for whom a poor prognosis was considered because of severe ADC reduction throughout the bihemispheric white matter accompanied by hemorrhagic TAI throughout the hemispheres and brainstem. We performed longitudinal comparisons of diffusion tensor imaging (DTI) data (which include DWI and ADC) that were acquired in association with standardized neurobehavioral assessment at four time points over the first year of recovery – day 8 (coma), day 44 (minimally conscious state), day 198 (post-traumatic confusional state), and day 366 (restoration of mental capacity and community reintegration). Finally, we map the patient’s traumatic microbleeds, detected with T2*-weighted imaging, onto a template of brainstem arousal nuclei to determine which components of the ascending reticular activating system (ARAS) were damaged by hemorrhagic axonal injury.
Methods
Clinical Data
A 19-year-old healthy right-handed man sustained a severe TBI with loss of consciousness as the result of a motor vehicle accident. The patient was the unrestrained driver of a sports utility vehicle that struck a telephone pole. He was found unresponsive in the front seat and extraction from the vehicle lasted 8 minutes, during which he was noted to have agonal respirations with a respiratory rate of 8 breaths per minute. Intubation could not be performed by emergency medical personnel at the scene of the crash due to severe facial fractures. On arrival to the Emergency Department at our institution 37 minutes after the accident, the patient was afebrile and hemodynamically stable (BP 177/90 mmHg, HR 115 beats per minute) with a pulse oximetry oxygen saturation of 88 to 91% while receiving bag-mask ventilation. He was intubated immediately. The first arterial blood gas analyzed at the time of intubation revealed a pH of 7.23, PCO2 of 66 mmHg, and PO2 of 43 mmHg. After intubation, the PO2 increased to > 80 mmHg for the remainder of his resuscitation.
Neurological examination demonstrated that although both arms were moving spontaneously, his eyes remained closed and he was unable to respond purposefully to verbal commands or noxious stimuli. The pupils were 3 mm and minimally reactive. Corneal reflexes could not be assessed due to periorbital injury. The cough reflex was present. Head CT scan revealed a left frontal epidural hematoma (maximal thickness of 24 mm), right frontotemporal epidural hematoma (maximal thickness = 10 mm), left frontotemporal subdural hematoma (maximal thickness = 6 mm), bilateral frontotemporal contusions, intraventricular hemorrhage, and multifocal punctate hyperdense lesions in the bihemispheric white matter suggesting hemorrhagic TAI. There was rightward midline shift of 7 mm at the level of the septum pellucidum. The basal cisterns and fourth ventricle were effaced, and there was downward herniation of the cerebellar tonsils into the foramen magnum. A depressed fracture of the left sphenoid triangle was present, as well as extensive bilateral facial fractures with possible compression injury to the optic nerves.
Within 54 minutes of arrival at the Emergency Department, the patient was taken to the operating room for left-sided decompressive hemicraniectomy, evacuation of the left frontal epidural hematoma, and placement of an intracranial pressure monitor (bolt). A post-operative head CT scan demonstrated interval enlargement of the right frontotemporal epidural hemorrhage (from 10 to 27 mm in maximal diameter) necessitating right-sided hemicraniectomy and right frontal epidural hematoma evacuation. Throughout the initial resuscitation and surgeries, the patient’s systolic BP remained above 90 mmHg and his mean arterial pressure was greater than 60 mmHg. He received a total of 6 units packed red blood cells within the first 24 hours of his injury, and his hemoglobin never fell below 7.0 g/dl during his hospitalization. Based on his neurological examination at the time of admission and his initial laboratory and CT data, the MRC CRASH model [18] predicted a 78% chance of an “unfavorable outcome” at 6 months and the IMPACT model [15] predicted a 30% chance of an unfavorable outcome at 6 months, with unfavorable outcome defined as a Glasgow Outcome Scale score of 1 (death), 2 (persistent vegetative state) or 3 (severe disability).
The early post-operative course (day 1 to 6) was notable for paroxysmal sympathetic hyperactivity [38], diabetes insipidus, electrographic seizures (intermittently associated with facial twitching), chest tube placement for left-sided pneumothorax, removal of the intracranial pressure monitor with concurrent placement of an external ventricular drain, and temporary external fixation of a left femur fracture. He was treated with antibiotics for pneumonia and a urinary tract infection, but there was no evidence of sepsis at any time during his hospitalization. On day 6, he underwent tracheostomy and gastrostomy tube placement.
The patient’s first MRI scan was performed on day 8, in part to assist in making a prognosis for recovery. At this time, the patient was in a coma (intubated and off sedation) with no eye opening, no response to commands, flexor posturing of the right arm and leg, and extensor posturing of the left arm and leg (Glasgow Coma Scale score = 5T [Eyes = 1, Motor = 3, Verbal = 1T]). The pupils were asymmetric and non-reactive to light (OD 3 mm, OS 4 mm), but the corneal and cough reflexes were present. Brain MRI revealed confluent, bihemispheric white matter hyperintensity on DWI with corresponding hypointensity on the ADC maps, suggesting restricted diffusion of water throughout the white matter (Fig. 1). The T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence showed hyperintensity in the same bihemispheric white matter distribution, though not as prominent as the DWI hyperintensity (Fig. 1). Widespread punctate hypointense lesions were seen in the bihemispheric white matter and brainstem on the T2* GRE sequence, indicating hemorrhagic TAI (Figs. 1 and 2). The presence of hemorrhagic TAI lesions in the corpus callosum and right dorsal pons were consistent with the most severe grade of TAI (i.e., “grade 3 diffuse axonal injury”) [24, 25]. Because of the imaging and clinical findings, the patient’s prognosis was judged to be poor. After extensive discussion over several days with the patient’s parents, the decision was made collectively to continue aggressive medical and surgical care.
Fig. 1.
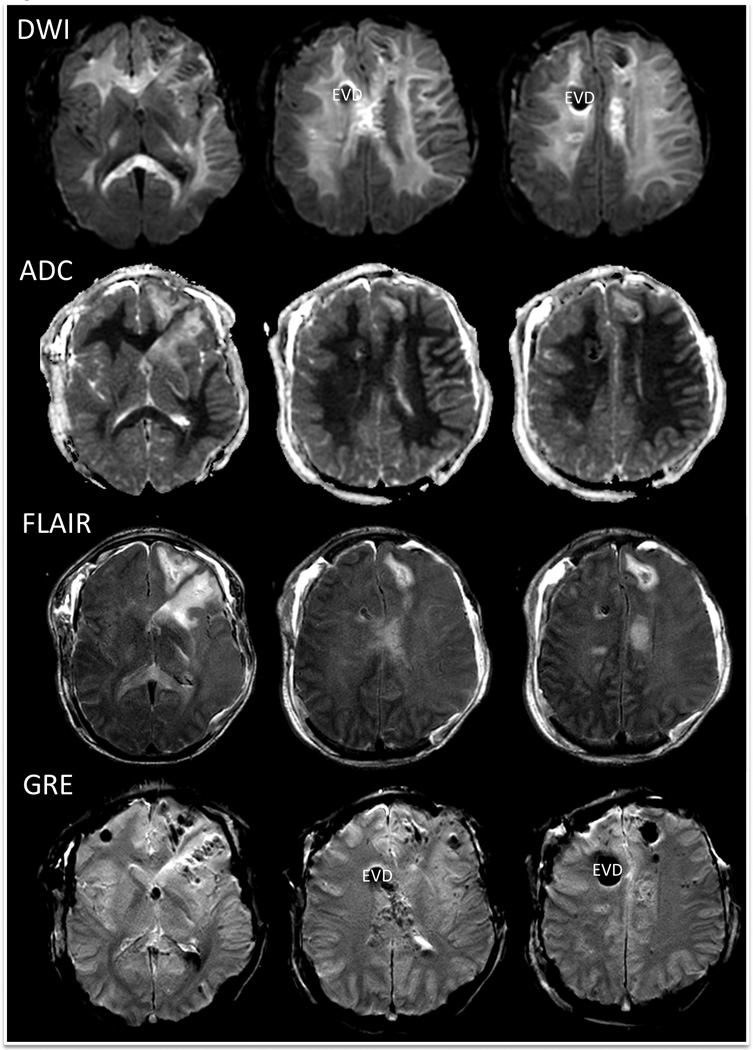
Day 8 MRI findings: diffusion-weighted images (DWI), apparent diffusion coefficient (ADC) maps, T2-weighted fluid attenuated inversion recovery (FLAIR) images, and T2* gradient-recalled echo (GRE) images. The hyperintense signal in the bihemispheric white matter seen on the DWI and FLAIR images, in association with the hypointense signal on the ADC maps, indicates restricted diffusion of water. The scattered, punctate, hypointense lesions on GRE indicate hemorrhagic traumatic axonal injury. Of note, the large right frontal hypointensity seen on the DWI and GRE images represents susceptibility artifact from an external ventricular drain (EVD).
Fig. 2.

Mapping traumatic microbleeds in the brainstem. Traumatic microbleeds are punctate hypointense lesions indicated by white arrows in the rostral pons (top row) and caudal midbrain (bottom row). In the right panel, traumatic microbleeds identified by 3 Tesla susceptibility-weighted imaging (SWI) are coregistered to Montreal Neurological Institute (MNI152) space and superimposed upon a template of brainstem arousal nuclei. Traumatic microbleeds are colored red, and brainstem arousal nuclei are color-coded as follows: dark blue, locus coeruleus; turquoise, dorsal raphe´; white, pontis oralis (pontine reticular formation); green, median raphe´; yellow, parabrachial nuclear complex; light blue, periaqueductal grey matter; pink, ventral tegmental area; purple, pedunculopontine nucleus. The traumatic microbleeds overlap partially with the right-sided locus coeruleus, parabrachial nuclear complex, dorsal raphe´, and periaqueductal grey matter. Neuroanatomic landmarks: Cb, cerebellum; 4th, fourth ventricle; CP, cerebral peduncle; Aq, cerebral acqueduct. Of note, although the GRE and SWI datasets were acquired on different days, the paramagnetic properties of blood are expected to produce the same hypointense signal at each time point.
Continuous electroencephalography (EEG) was initially performed from day 2 to 3 because of episodes of tachycardia and upper extremity posturing. No epileptiform activity was identified at this time; rather, the EEG revealed generalized low-amplitude delta slowing with no electrographic reactivity to tactile or auditory stimulation. Continuous EEG was repeated from day 8 to day 15 because of persistent coma and episodes of facial twitching. Non-convulsive status epilepticus from a left frontal seizure focus was detected on day 12, and the patient was treated with propofol-induced burst suppression for 24 hours. On day 17, the patient began to spontaneously open his eyes without demonstrating awareness of self or environment, indicating transition from coma to the vegetative state [4]. A repeat EEG on day 17 also demonstrated electrographic reactivity to stimulation for the first time. During week 4, the patient’s eyes were open intermittently, but he did not regard or track the examiner. Spontaneous movements were observed in the bilateral upper extremities and right lower extremity, and triple flexion was observed in the left lower extremity. During week 5, the patient intermittently exhibited episodes of visual fixation and localization to noxious stimuli with the upper extremities, signaling early emergence of consciousness and transition to the minimally conscious state [5].
A follow-up MRI scan performed on day 44 demonstrated interval normalization of the bihemispheric white matter signal on DWI and a corresponding increase in diffusivity on the ADC maps (Fig. 3). On days 36 and 50, he underwent surgical repair of multiple skull-base fractures. On day 59, the patient was transferred to an inpatient disorders of consciousness program for comprehensive neurorehabilitation, where he showed continued behavioral evidence of recovery. On day 60, he regained the ability to mobilize in bed with cues. Specifically, he could transition from the supine to sitting position and vice versa with minimal physical assistance by one therapist. In addition, he transferred from bed to a chair with moderate assistance by two therapists. He was correctly answering biographical questions approximately 75% of the time and following one-step commands approximately 40% of the time. He answered “yes” with thumbs-up and “no” with horizontal head turning. On day 70, amantadine hydrochloride was initiated at 100 mg twice daily (7AM and 1PM) and subsequently titrated up to 125mg twice daily to promote arousal and behavioral responsiveness. At the time of initiation of this therapy, the patient could stand on his own by placing his hand on a walker. He required minimal assistance with transfers and was able to ambulate fifteen feet with the therapist’s hands supporting his hips for balance. He also began phonating verbal responses to commands approximately 50% of the time and was following one-step commands with 70% accuracy. Amantadine hydrochloride was discontinued on day 77 after a series of febrile infections (pneumonia and clostridium difficile colitis) confounded the assessment of this medication’s efficacy. By day 85, the infections had been treated, and methylphenidate was initiated at 2.5mg twice daily (7AM and 1PM). The patient was now identifying words and objects inconsistently via eye gaze and assisting with self-care activities. On day 90, he was able to ambulate 50 feet. He responded to specific yes-no questions 75% of the time with 60% accuracy.
Fig. 3.
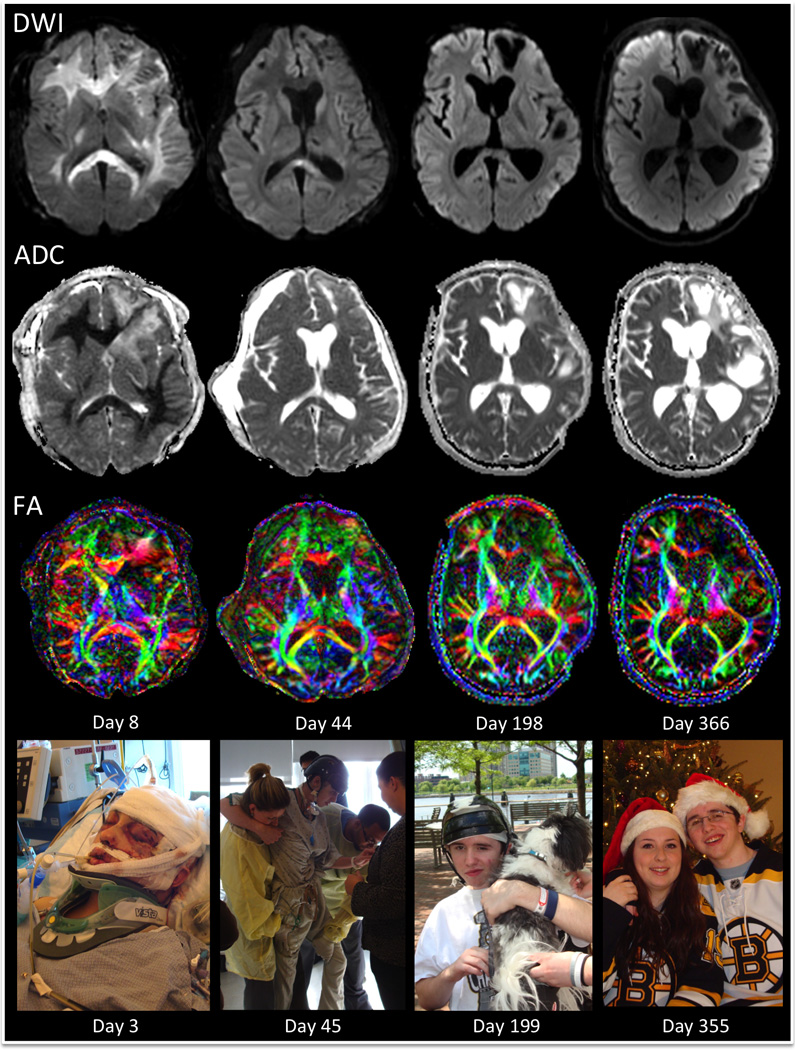
Longitudinal MRI Findings: diffusion-weighted images (DWI), apparent diffusion coefficient (ADC) maps, and color fractional anisotropy (FA) maps. The bihemispheric white matter hyperintensity seen on DWI and the corresponding hypointensity seen on ADC on day 8 are mostly resolved by day 44 and are absent by day 198. The color FA maps demonstrate preservation of multiple white matter pathways in the cerebral hemispheres, despite the presence of extensive diffusion restriction on day 8. Fiber orientations are color-coded according to convention: red, medial-lateral; green, anterior-posterior; blue, superior-inferior. Encephalomalacia is seen on day 198 and 366 in the left frontotemporal regions that were acutely affected by contusions. Photographs of the patient during each stage of his recovery are shown in the bottom row at time points that correspond closely to the timing of the MRI scans. All photographs are provided by the patient’s family and shown with consent.
A third MRI scan was obtained prospectively on day 198. Neurobehavioral assessment indicated that the patient had emerged from the minimally conscious state and met criteria for the post-traumatic confusional state [39]. By the fourth scan, performed on day 366, he was consistently oriented to his location, and the date, year, and day of the week. He was living at home with his family and was independent in most activities of daily living, such as feeding and toileting, although he still required minimal assistance with grooming. The Disability Rating Scale (DRS) [40] was administered within three days of each scan: on the same day as the first scan, the day before the second scan, three days before the third scan, and on the day of the last scan. The DRS score incorporates assessments of arousal and awareness, ability to perform self-care, level of physical dependence, and psychosocial capacity for work or school [40]. Total DRS scores range from 0 (no disability) to 30 (death), and the DRS may be more sensitive to detecting functional recovery after TBI than the Glasgow Outcome Scale [41] or the Functional Independence Measure [42]. A final DRS score was assessed 2 years post-injury, at which time the patient was functionally independent within his home environment and was also performing basic tasks in a supervised work environment.
Image Acquisition
All diffusion data were acquired on 1.5 Tesla MRI scanners utilizing an echo-planar twice-refocused spin-echo sequence [43] with 25 directional diffusion gradients and diffusion-weighting (b-value) of 1000 s/mm2. The first two scans were performed on a General Electric Signa HDxt scanner (General Electric Medical Systems, Waukesha, WI), one of our inpatient clinical scanners; DTI data are acquired as part of the routine MRI protocol at our institution. The third and fourth scans were performed on a Siemens Avanto scanner (Siemens Medical Solutions, Erlangen, Germany) at our outpatient facility as part of a prospective research protocol approved by our institutional review board, with the family’s consent and the patient’s assent. The diffusion-tensor sequence for the first two scans utilized the following parameters: TR = 5000 msec, TE = 98.8 to 105.9 msec, acquisition matrix = 128 × 128 (zero-filled to 256 × 256), field-of-view = 230 mm, in-plane resolution = 0.9 × 0.9 mm, and slice thickness = 5 mm with a 1 mm interslice gap. Three non-diffusion-weighted volumes with b-value = 0 s/mm2 (b0) were acquired. The diffusion tensor sequence for the third and fourth scans utilized the following parameters: TR = 5000 msec, TE = 88 msec, acquisition matrix = 128 × 128, field-of-view = 220 mm, in-plane resolution = 1.72 × 1.72 mm, and slice thickness = 5 mm with a 1 to 1.5 mm interslice gap. Five b0 volumes were acquired.
The axial T2*-weighted 2D GRE echo-planar imaging sequence performed during the first scan (day 8) utilized the following parameters: TR = 12000 msec, TE = 29.7 msec, flip angle = 90 degrees, slice thickness = 5 mm with a 1 mm gap, 256 × 256 acquisition matrix, field-of-view = 230 mm, in-plane resolution 0.9 × 0.9 mm. Axial 3D susceptibility-weighted imaging (SWI) was performed on Siemens 1.5 Tesla Avanto and 3 Tesla Trio MRI scanners at the fourth time point (day 366) utilizing the following parameters: TR = 27ms, TE = 20 ms, flip angle = 15 degrees, 182 × 256 acquisition matrix at 95% sampling (192 × 256 matrix), field-of-view = 172 × 230 mm, in-plane resolution = 0.9 × 0.9 mm2, and 1.5 mm slice thickness.
MRI and DTI Analysis
Diffusion data were processed using Diffusion Toolkit version 6.2.1 and analyzed for ADC and FA values using Trackvis version 5.2.1 (Wang and Wedeen, Athinoula A. Martinos Center for Biomedical Imaging, www.trackvis.org). Regions of interest (ROIs) were manually traced on the b0 volumes for each scan to avoid biasing ROI neuroanatomic localization by the ADC or FA findings. These non-diffusion weighted b0 volumes are inherently coregistered to the ADC and FA maps. In order to minimize the likelihood of obtaining spurious results due to multiple comparisons, we focused our analyses on ROIs that have been shown in prior MRI and/or DTI studies to have prognostic utility or to correlate with recovery of consciousness after severe brain injury. Specifically, the genu and splenium of the corpus callosum were chosen because of studies showing an association between FA in these regions and outcome after TBI [44–46]. In addition, the subcortical white matter and thalamus were chosen in view of evidence demonstrating that ADC values in these regions differ between patients in the vegetative and minimally conscious states [47]. The entire genu, splenium, and thalamus were manually outlined for the purpose of ADC and FA analysis. For the subcortical white matter analysis, the bihemispheric white matter was outlined in a single axial slice at the level of the centrum semiovale and the body of the corpus callosum (middle column in Fig. 1). This axial slice was chosen as a representative section of subcortical white matter because it was superior to the region of severe atrophy related to the left frontotemporal contusions and it was inferior to the region that was most affected by susceptibility artifact from the external ventricular drain. Voxels that were affected by volume-averaging of signal from the cerebrospinal fluid were rigorously excluded.
To analyze the extent and severity of hemorrhagic TAI, the T2* GRE data from day 8 were compared to the SWI data from the 1.5 Tesla and 3 Tesla scans performed on day 366. The scan with the greatest number of traumatic microbleeds (3 Tesla SWI on day 366) was then transformed to standard Montreal Neurological Institute (MNI) space using the MNI152 T1-weighted dataset as a template. To improve the accuracy of the coregistration, the skull was stripped from the 3 Tesla SWI dataset using the Brain Extraction Tool in the FMRIB Software Library (FSL) [48]. Subsequently, the 3 Tesla SWI dataset was coregistered to the MNI152 template using FMRIB’s Linear Image Registration Tool (FLIRT version 5.5) in FSL [48] with an affine transformation. After coregistration, the traumatic microbleeds in the patient’s brainstem were traced in MNI space and their neuroanatomic location was mapped onto a template of ARAS brainstem nuclei. This template of the brainstem nuclei that are implicated in arousal (wakefulness), and hence consciousness [49–51], was manually traced in MNI space based on data from a recent histo-radiologic correlation study of the ARAS arousal nuclei (Fig. 2) [51].
Results
Longitudinal MRI findings are shown in Figure 3, and longitudinal quantitative ADC and FA measurements for each ROI are reported in Table 1. Mean ADC values on day 8 in the white matter of the genu, splenium, and subcortical white matter ranged from 230 to 400 × 10−6 mm2/sec (Table 1). These ADC values were in a range that is believed to indicate irreversible neuronal injury (below 600 × 10−6 mm2/sec) [52, 53]. Mean ADC values in the white matter ROIs increased at subsequent time points (except in the subcortical white matter between day 198 and day 366), with the largest interval increase occurring between days 8 and 44. FA values in the white matter ROIs were reduced at each time point compared to most published normal values [47, 54–56]. A decline in FA was observed for each white matter ROI at each time point between day 8 and day 366 (except in the subcortical white matter between day 198 and day 366), although the magnitude and rate of decline varied. The largest FA decline between day 8 and day 366 was present in the genu of the corpus callosum (from 0.67 +/− 0.17 to 0.35 +/− 0.07, − 48%). In the thalamic ROI, mean ADC and mean FA values were similar to published normal values at each time point [47, 54, 57]. Although the subcortical white matter diffusion restriction was profound, diffusion restriction was not observed in the cerebral cortex on day 8. Quantitative longitudinal analysis of ADC and FA values within the cerebral cortex was precluded by the emergence of extensive encephalomalacia in the frontotemporal regions affected by contusions.
Table 1.
Mean apparent diffusion coefficient and fractional anisotropy values for the subcortical white matter, genu of the corpus callosum, splenium of the corpus callosum, and thalami at each time point.
| Region of Interest |
Mean ADC (x 10−6 mm2/s) | Mean FA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 8 | Day 44 | Day 198 | Day 366 | % Change Day 8 to 366 |
Day 8 | Day 44 | Day 198 | Day 366 | % Change Day 8 to 366 |
|
| Subcortical White Matter |
370 ± 140 | 770 ± 120 | 920 ± 180 | 890 ± 170 | + 141% | 0.41 ± 0.14 | 0.39 ± 0.13 | 0.32 ± 0.11 | 0.33 ± 0.11 | − 20% |
| Genu CC |
230 ± 80 | 920 ± 130 | 970 ± 110 | 1100 ± 210 | + 378% | 0.67 ± 0.17 | 0.45 ± 0.09 | 0.39 ± 0.09 | 0.35 ± 0.07 | − 48% |
| Splenium CC |
400 ± 160 | 810 ± 200 | 1000 ± 170 | 1040 ± 200 | + 160% | 0.54 ± 0.15 | 0.53 ± 0.13 | 0.47 ± 0.14 | 0.43 ± 0.10 | − 20% |
| Thalami | 770 ± 90 | 730 ± 90 | 860 ± 110 | 890 ± 180 | + 16% | 0.39 ± 0.10 | 0.35 ± 0.11 | 0.35 ± 0.08 | 0.37 ± 0.08 | − 5% |
Data are reported as mean +/− standard deviation.
Abbreviations: ADC, apparent diffusion coefficient; CC, corpus callosum; FA, fractional anisotropy.
Four brainstem traumatic microbleeds, indicating hemorrhagic TAI, were identified on the 3 Tesla SWI sequence on day 366: two in the rostral pons and two in the caudal midbrain (all right-sided). In comparison, three traumatic microbleeds were identified on the 1.5 Tesla SWI sequence on day 366 and one traumatic microbleed with a multi-lobed appearance was observed on the 1.5 Tesla GRE sequence on day 8. The 1.5 Tesla GRE sequence did not detect any midbrain microbleeds, and the 1.5 Tesla SWI sequence only detected one midbrain microbleed. Neuroanatomic localization of the brainstem traumatic microbleeds on the ARAS template revealed that the hemorrhagic axonal injury involved the right-sided locus coeruleus, dorsal raphe´, parabrachial nuclear complex and periaqueductal grey matter (Fig. 2).
The patient’s longitudinal DRS scores are shown in Table 2. His DRS score declined, indicating functional improvement, at each follow-up assessment. His DRS score was 8 at the time of the final MRI scan on day 366, consistent with moderately severe functional disability. At the 2-year follow-up, his DRS score had improved further to 3, indicating partial residual disability (see also supplementary video).
Table 2.
Longitudinal improvements in the patient’s level of consciousness and degree of disability on the Disability Rating Scale score at the time of each MRI scan.
| Clinical Assessment |
1 Week (Day 8) |
1.5 Months (Day 44) |
6.5 Months (Day 198) |
1 Year (Day 366) |
2 Years (Day 738) |
|---|---|---|---|---|---|
| Level of Consciousness & Function |
Coma | Minimally conscious state |
Post-traumatic confusional state |
Independence in ADLs |
Functional independence in home environment |
| DRS Score and Disability Category |
27 * |
21 Extremely Severe Disability |
15 Severe Disability |
8 Moderately Severe Disability |
3 Partial Disability |
Decreasing scores on the Disability Rating Scale (DRS) are associated with decreasing degrees of disability in daily function.
No disability category is listed because the category corresponding to a DRS score of 27 is “extreme vegetative state,” which does not accurately reflect the patient’s comatose state on day 8.
Discussion
In this 19-year-old man with severe TBI causing coma, the early MRI data incorrectly suggested a poor prognosis. Despite the presence of brainstem hemorrhagic axonal injury on T2*-weighted GRE, our patient showed marked improvements in arousal and attention within 6 weeks of onset. Furthermore, despite extensive bihemispheric diffusion restriction on the ADC maps, our patient experienced cognitive and functional recovery sufficient to support independent living in the home environment. This recovery continued for the first two years of follow-up, with associated dynamic changes in white matter ADC and FA values observed on serial neuroimaging. The longitudinal clinical-radiologic observations in this case thus demonstrate that recovery of meaningful function is possible even when MRI data suggest a highly unfavorable prognosis. Although recent studies show that MRI [7, 19, 20, 22, 26, 58, 59], and in particular DTI [60], is a powerful predictor of outcome after severe brain injury, our study suggests that early (i.e., day 8) MRI may have limited specificity for predicting poor outcome.
Expectations for recovery of communication and self-directed behavior were considerably lower than those for recovery of arousal, given that the bilateral diffusion restriction encompassed nearly the entire hemispheric white matter, while the brainstem injury was limited to the right side. Reversal of restricted diffusion in TAI has been described in rare reports [61, 62], but to our knowledge this type of reversal has not been previously described with serial neuroimaging or in a case with such a widespread extent of axonal injury. Notably, confluent white matter restricted diffusion in head trauma is rare and may indicate superimposed hypoxic injury. Indeed, the presumed period of hypoxia that occurred during the patient’s prolonged extraction from his car (supported by observations of agonal breathing by emergency responders), suggests that the pattern of injury observed on MRI may have been caused by hypoxic cerebral injury superimposed upon TAI. Furthermore, the pattern of injury observed by MRI on day 8 was similar to that described during the same time period (i.e., day 6–12) in patients with hypoxic-ischemic injury after cardiac arrest [63]. While prior studies of patients in coma following hypoxic-ischemic injury have indicated that median whole-brain ADC values of less than approximately 600 × 10−6 mm2/sec are associated with poor outcome [33], our patient’s recovery suggests that TAI, or TAI in combination with hypoxia, can cause diffusion restriction via a distinct – and possibly reversible – set of pathophysiological mechanisms. Furthermore, the absence of cortical necrosis on longitudinal imaging analysis is consistent with the hypothesized injury mechanisms of TAI and hypoxia, without concurrent ischemia. Indeed, patients with isolated hypoxia may have greater potential for neurologic recovery than those with both hypoxia and ischemia [64, 65].
Potential mechanisms for intracellular diffusion restriction in TAI include impaired axoplasmic transport due to neurofilament disruption, loss of transmembrane ionic homeostasis, free radical formation and/or glutamate-induced excitotoxicity [28]. In addition, extracellular diffusion restriction may occur as axonal membrane fragmentation and hemorrhage create barriers to water diffusion [59]. These heterogeneous pathophysiologic mechanisms in TAI may help to explain the variable time course of diffusion restriction in TAI. DWI hyperintensity and ADC hypointensity have been reported up to 18 days after TAI [30], as compared to the stereotypical pseudonormalization of restricted diffusion by day 7–10 in ischemia [52, 66]. Furthermore, animal models of TBI have demonstrated that axonal injury may be non-disruptive in that the axonal membrane and/or neurofilamentous architecture may be disturbed without complete transection of the axon [67, 68]. It is presumably these incompletely injured axons that enable recovery of function in cases like the one described here.
Notably, ADC and FA values in the thalami were relatively preserved both at day 8 and one year after the patient’s injury. Studies of comatose cardiac-arrest patients have suggested an association between reduced thalamic ADC values and poor outcomes [33, 69], and histopathologic studies have implicated thalamic injury in the pathogenesis of prolonged altered consciousness [70, 71]. The potential sparing of the thalami, as indicated by near-normal ADC and FA values at each time point, may have thus portended a good outcome in this patient. DTI studies of thalamic FA in other diseases that affect the white matter, such as multiple sclerosis, have found that hemispheric white matter injury may be paradoxically associated with increased thalamic FA [57]. The etiology of this increase in thalamic FA is incompletely understood but may reflect loss of intra-thalamic crossing fibers due to Wallerian degeneration of afferent and/or efferent white matter pathways entering/exiting the thalamus. Further studies are therefore necessary to elucidate the relationship between TAI and thalamic FA and ADC to determine whether these quantitative imaging biomarkers have prognostic utility in patients with severe TBI.
The limitations of early MRI for predicting outcome following severe TBI and coma are further highlighted by the localization of the patient’s brainstem microbleeds. Currently, brainstem lesions associated with TAI are classified according to the three-tiered grading system developed by Adams and colleagues for histopathological grading of diffuse axonal injury [25]. This approach has been widely applied to MRI studies of TAI because it provides an easily identifiable classifier of TAI severity. Yet, this three-tiered system of TAI grading (in which our patient would be graded as having “grade 3 diffuse axonal injury”) does not distinguish between brainstem lesions that affect the ARAS arousal pathways and lesions outside arousal pathways that are less likely to impact recovery of consciousness. In our patient, brainstem microbleeds were present within the locus coeruleus, dorsal raphe´, parabrachial nuclear complex, and periaqueductal grey matter, but each of these nuclei was only partially affected by small microbleeds and only on the right side of the brainstem. Hence, it appears that partial, unilateral injury to these nuclei, which are a source of noradrenergic, serotonergic, glutamatergic and dopaminergic neuronal projections to the diencephalon and forebrain [50], does not prevent recovery of arousal when the arousal pathways of the contralateral, homologous nuclei and other ipsilateral arousal-related nuclei are preserved.
It remains to be determined which nuclei of the ARAS are most important for recovery of consciousness. Nevertheless, a rigorous approach to neuroanatomic localization of brainstem lesions in traumatic coma is likely to improve the description of patients with TBI enrolled in clinical trials. Recovery of arousal has been described in MRI studies of patients with unilateral brainstem TAI [7] and patients with unilateral ischemic lesions in the brainstem [49]. These findings suggest that the brainstem arousal network contains redundant circuitry, a hypothesis that is supported by a recent histo-radiological connectivity analysis in which several of the known neurotransmitter-specific brainstem arousal nuclei were shown to connect with overlapping sites in the thalamus, hypothalamus, and basal forebrain [51]. It is also possible that ARAS lesion mapping may be used in the future to guide individualized neurostimulant therapies that will target specific neurotransmitter networks affected by TAI. In this case, it is not possible to discern the relationship between initiation of the neurostimulants (i.e., amantadine hydrochloride and methylphenidate) and the patient’s clinical status. Both medications were started during the subacute period when spontaneous recovery is most rapid and the patient had already demonstrated clear-cut behavioral signs of improvement. Under these circumstances, one can only speculate about the impact of these medications on the patient’s recovery.
Finally, our findings are consistent with prior studies suggesting that SWI is more sensitive than 2D–GRE (with 5 mm thick slices) for detecting brainstem microbleeds [72] at 1.5 Tesla, and that higher field strength (e.g., 3 Tesla MRI) using thinner slices (e.g., 1.5 mm) further increases the sensitivity for detecting microbleeds [73]. Although the GRE and SWI data were acquired on different days, the paramagnetic properties of blood are expected to produce the same hypointense signal characteristics at each time point. Moreover, there is no indication from the clinical data that the patient experienced a new brainstem hemorrhage between days 8 and 366.
A potential limitation of our study is the utilization of different MRI scanners and slightly different diffusion sequences for the first and second scans, as compared to the third and fourth scans. Nonetheless, the diffusion sequences that were used to measure ADC and FA at each time point utilized the same number of directional diffusion gradients (n=25) and the same b-value (1000 sec/mm2). Given that these are the two parameters most likely to affect the quantitative diffusion measurements [74, 75], it is unlikely that other small differences between the two diffusion sequences would have significantly confounded the results of the longitudinal imaging analysis. It is also notable that our patient was a healthy young man who received prompt transport to a nearby trauma center and neurocritical care unit, as well as rapid resuscitation and surgical evacuation of intracranial mass lesions. While age is currently recognized as an important prognostic factor and is incorporated in the IMPACT and MRC CRASH prognostic models [15, 18], it is important to consider that our patient’s healthy pre-injury status and rapid treatment guided by physician and nurse specialists in neurocritical care may have influenced his recovery in ways that are not measured by current prognostic models. Finally, there are additional sequences available on clinical MRI scanners, such as magnetic resonance spectroscopy, that were not performed in this study but that may have provided prognostic utility [76].
Conclusions
Our patient’s unexpected recovery of consciousness, communication, and functional independence suggests that MRI data must be interpreted with caution in the early stage of traumatic coma. The correlative clinical-radiologic observations in this case highlight current limitations of early MRI as a prognostic tool following TBI and reinforce the need for ongoing development of multimodal models to predict outcomes for patients with traumatic coma.
Supplementary Material
The first two years of the patient’s recovery are documented longitudinally in pictures and videos that were shared by the patient and his family with their consent. All clinicians who appear in the video also provided consent. An interview with the patient was performed on day 783, at which time he reflected on the major milestones in his recovery.
Acknowledgments
We are grateful to the patient and his family for participating in this study and for sharing their photos and videos. All photos and videos are shown with consent. We thank Kristin Parlman and Anne McGrail for their assistance with functional outcome assessments. We also thank the nurses and the physical, occupational, and speech/language therapy teams at Massachusetts General Hospital, Spaulding Rehabilitation Hospital, and Crotched Mountain Rehabilitation Center. We thank Kathryn R. Tringale and Brittany Sorice for assistance with video capture and editing. We are grateful to Dr. Hannah C. Kinney for consultation regarding neuroanatomic localization of the regions of interest used in this study.
Funding This study was supported in part by grants from the National Institutes of Health (R25NS065743 and P41EB015896), the National Institute on Disability and Rehabilitation Research (H133A120085), and the Center for Integration of Medicine and Innovative Technology (Boston, MA). The views in this article represent those of the authors alone, and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. Government.
Footnotes
Electronic supplementary material:
This manuscript contains one supplementary video file.
Conflict of interest None.
Contributor Information
Brian L. Edlow, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA.
Joseph T. Giacino, Department of Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, MA; Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Ronald E. Hirschberg, Department of Physical Medicine and Rehabilitation, Spaulding Rehabilitation Hospital, Harvard Medical School, Boston, MA Department of Physical Medicine and Rehabilitation, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Jason Gerrard, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Ona Wu, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA; Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Leigh R. Hochberg, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA; School of Engineering and Institute for Brain Science, Brown University; Center for Neurorestoration and Neurotechnology, Dept. Veterans Affairs Medical Center, Providence, RI.
References
- 1.Ling GS, Marshall SA. Management of traumatic brain injury in the intensive care unit. Neurol Clin. 2008;26:409–426. doi: 10.1016/j.ncl.2008.02.001. viii. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta (GA): N.C.f.I.P. Centers for Disease Control and Prevention, Editor 2006; [Google Scholar]
- 3.Selassie AW, Zaloshnja E, Langlois JA, et al. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- 4.Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet. 1972;1:734–737. doi: 10.1016/s0140-6736(72)90242-5. [DOI] [PubMed] [Google Scholar]
- 5.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 6.Katz DI, Polyak M, Coughlan D, et al. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog Brain Res. 2009;177:73–88. doi: 10.1016/S0079-6123(09)17707-5. [DOI] [PubMed] [Google Scholar]
- 7.Skandsen T, Kvistad KA, Solheim O, et al. Prognostic value of magnetic resonance imaging in moderate and severe head injury: a prospective study of early MRI findings and one-year outcome. J Neurotrauma. 2011;28:691–699. doi: 10.1089/neu.2010.1590. [DOI] [PubMed] [Google Scholar]
- 8.Gennarelli TA, Spielman GM, Langfitt TW, et al. Influence of the type of intracranial lesion on outcome from severe head injury. J Neurosurg. 1982;56:26–32. doi: 10.3171/jns.1982.56.1.0026. [DOI] [PubMed] [Google Scholar]
- 9.Bell RS, Vo AH, Neal CJ, et al. Military traumatic brain and spinal column injury: a 5-year study of the impact blast and other military grade weaponry on the central nervous system. J Trauma. 2009;66:S104–S111. doi: 10.1097/TA.0b013e31819d88c8. [DOI] [PubMed] [Google Scholar]
- 10.Estraneo A, Moretta P, Loreto V, et al. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010;75:239–245. doi: 10.1212/WNL.0b013e3181e8e8cc. [DOI] [PubMed] [Google Scholar]
- 11.Voss HU, Uluc AM, Dyke JP, et al. Possible axonal regrowth in late recovery from the minimally conscious state. J Clin Invest. 2006;116:2005–2011. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luaute J, Maucort-Boulch D, Tell L, et al. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology. 2010;75:246–252. doi: 10.1212/WNL.0b013e3181e8e8df. [DOI] [PubMed] [Google Scholar]
- 13.Nakase-Richardson R, Whyte J, Giacino JT, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma. 2012;29:59–65. doi: 10.1089/neu.2011.1829. [DOI] [PubMed] [Google Scholar]
- 14.Lammi MH, Smith VH, Tate RL, Taylor CM. The minimally conscious state and recovery potential: a follow-up study 2 to 5 years after traumatic brain injury. Arch Phys Med Rehabil. 2005;86:746–754. doi: 10.1016/j.apmr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Maas AI, Steyerberg EW, Butcher I, et al. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:303–314. doi: 10.1089/neu.2006.0033. [DOI] [PubMed] [Google Scholar]
- 16.Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:329–337. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 18.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagares A, Ramos A, Perez-Nunez A, et al. The role of MR imaging in assessing prognosis after severe and moderate head injury. Acta Neurochir (Wien) 2009;151:341–356. doi: 10.1007/s00701-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 20.Firsching R, Woischneck D, Diedrich M, et al. Early magnetic resonance imaging of brainstem lesions after severe head injury. J Neurosurg. 1998;89:707–712. doi: 10.3171/jns.1998.89.5.0707. [DOI] [PubMed] [Google Scholar]
- 21.Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol. 1988;150:673–682. doi: 10.2214/ajr.150.3.673. [DOI] [PubMed] [Google Scholar]
- 22.Paterakis K, Karantanas AH, Komnos A, Volikas Z. Outcome of patients with diffuse axonal injury: the significance and prognostic value of MRI in the acute phase. J Trauma. 2000;49:1071–1075. doi: 10.1097/00005373-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Gentry LR, Godersky JC, Thompson BH. Traumatic brain stem injury: MR imaging. Radiology. 1989;171:177–187. doi: 10.1148/radiology.171.1.2928523. [DOI] [PubMed] [Google Scholar]
- 24.Gentry LR. Imaging of closed head injury. Radiology. 1994;191:1–17. doi: 10.1148/radiology.191.1.8134551. [DOI] [PubMed] [Google Scholar]
- 25.Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 26.Kampfl A, Schmutzhard E, Franz G, et al. Prediction of recovery from post-traumatic vegetative state with cerebral magnetic-resonance imaging. Lancet. 1998;351:1763–1767. doi: 10.1016/S0140-6736(97)10301-4. [DOI] [PubMed] [Google Scholar]
- 27.Edlow BL, Wu O. Advanced neuroimaging in traumatic brain injury. Semin Neurol. 2012;32:374–400. doi: 10.1055/s-0032-1331810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barzo P, Marmarou A, Fatouros P, et al. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg. 1997;87:900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- 29.Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25:370–376. [PMC free article] [PubMed] [Google Scholar]
- 30.Liu AY, Maldjian JA, Bagley LJ, et al. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR Am J Neuroradiol. 1999;20:1636–1641. [PMC free article] [PubMed] [Google Scholar]
- 31.Betz J, Zhuo J, Roy A, et al. Prognostic value of diffusion tensor imaging parameters in severe traumatic brain injury. J Neurotrauma. 2012;29:1292–1305. doi: 10.1089/neu.2011.2215. [DOI] [PubMed] [Google Scholar]
- 32.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 33.Wu O, Sorensen AG, Benner T, et al. Comatose patients with cardiac arrest: predicting clinical outcome with diffusion-weighted MR imaging. Radiology. 2009;252:173–181. doi: 10.1148/radiol.2521081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijman CA, Mlynash M, Caulfield AF, et al. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Annals of Neurology. 2009;65:394–402. doi: 10.1002/ana.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakahara M, Ericson K, Bellander BM. Diffusion-weighted MR and apparent diffusion coefficient in the evaluation of severe brain injury. Acta Radiol. 2001;42:365–369. doi: 10.1080/028418501127346990. [DOI] [PubMed] [Google Scholar]
- 36.Scheid R, Preul C, Gruber O, et al. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am J Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- 37.Yanagawa Y, Tsushima Y, Tokumaru A, et al. A quantitative analysis of head injury using T2*-weighted gradient-echo imaging. J Trauma. 2000;49:272–277. doi: 10.1097/00005373-200008000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Hinson HE, Sheth KN. Manifestations of the hyperadrenergic state after acute brain injury. Curr Opin Crit Care. 2012;18:139–145. doi: 10.1097/MCC.0b013e3283513290. [DOI] [PubMed] [Google Scholar]
- 39.Stuss DT, Binns MA, Carruth FG, et al. The acute period of recovery from traumatic brain injury: posttraumatic amnesia or posttraumatic confusional state? J Neurosurg. 1999;90:635–643. doi: 10.3171/jns.1999.90.4.0635. [DOI] [PubMed] [Google Scholar]
- 40.Rappaport M, Hall KM, Hopkins K, et al. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118–123. [PubMed] [Google Scholar]
- 41.Hall K, Cope DN, Rappaport M. Glasgow Outcome Scale and Disability Rating Scale: comparative usefulness in following recovery in traumatic head injury. Arch Phys Med Rehabil. 1985;66:35–37. [PubMed] [Google Scholar]
- 42.Hammond FM, Grattan KD, Sasser H, et al. Long-term recovery course after traumatic brain injury: a comparison of the functional independence measure and disability rating scale. J Head Trauma Rehabil. 2001;16:318–329. doi: 10.1097/00001199-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 44.Sidaros A, Engberg AW, Sidaros K, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 45.Kumar R, Gupta RK, Husain M, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Inj. 2009;23:675–685. doi: 10.1080/02699050903014915. [DOI] [PubMed] [Google Scholar]
- 46.Rutgers DR, Fillard P, Paradot G, et al. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:1730–1735. doi: 10.3174/ajnr.A1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Espejo D, Bekinschtein T, Monti MM, et al. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage. 2011;54:103–112. doi: 10.1016/j.neuroimage.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 48.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 49.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–1536. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- 50.Parvizi J, Damasio A. Consciousness and the brainstem. Cognition. 2001;79:135–160. doi: 10.1016/s0010-0277(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 51.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71:531–546. doi: 10.1097/NEN.0b013e3182588293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlaug G, Siewert B, Benfield A, et al. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology. 1997;49:113–119. doi: 10.1212/wnl.49.1.113. [DOI] [PubMed] [Google Scholar]
- 53.Grandin C, Hermoye L, Duprez T, et al. Is there an ADC threshold predicting irreversible infarction in hyperacute stroke? Journal of Neuroradiology. 2002;29:68–71. [Google Scholar]
- 54.Sorensen AG, Wu O, Copen WA, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology. 1999;212:785–792. doi: 10.1148/radiology.212.3.r99se24785. [DOI] [PubMed] [Google Scholar]
- 55.Kraus MF, Susmaras T, Caughlin BP, et al. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 56.Kumar R, Husain M, Gupta RK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma. 2009;26:481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- 57.Tovar-Moll F, Evangelou IE, Chiu AW, et al. Thalamic involvement and its impact on clinical disability in patients with multiple sclerosis: a diffusion tensor imaging study at 3T. AJNR Am J Neuroradiol. 2009;30:1380–1386. doi: 10.3174/ajnr.A1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaefer PW, Huisman TA, Sorensen AG, et al. Diffusion-weighted MR imaging in closed head injury: high correlation with initial glasgow coma scale score and score on modified Rankin scale at discharge. Radiology. 2004;233:58–66. doi: 10.1148/radiol.2323031173. [DOI] [PubMed] [Google Scholar]
- 59.Zheng WB, Liu GR, Li LP, Wu RH. Prediction of recovery from a post-traumatic coma state by diffusion-weighted imaging (DWI) in patients with diffuse axonal injury. Neuroradiology. 2007;49:271–279. doi: 10.1007/s00234-006-0187-8. [DOI] [PubMed] [Google Scholar]
- 60.Galanaud D, Perlbarg V, Gupta R, et al. Assessment of white matter injury and outcome in severe brain trauma: a prospective multicenter cohort. Anesthesiology. 2012;117:1300–1310. doi: 10.1097/ALN.0b013e3182755558. [DOI] [PubMed] [Google Scholar]
- 61.Muccio CF, De Simone M, Esposito G, et al. Reversible post-traumatic bilateral extensive restricted diffusion of the brain. A case study and review of the literature. Brain Inj. 2009;23:466–472. doi: 10.1080/02699050902841912. [DOI] [PubMed] [Google Scholar]
- 62.Takayama H, Kobayashi M, Sugishita M, Mihara B. Diffusion-weighted imaging demonstrates transient cytotoxic edema involving the corpus callosum in a patient with diffuse brain injury. Clin Neurol Neurosurg. 2000;102:135–139. doi: 10.1016/s0303-8467(00)00079-2. [DOI] [PubMed] [Google Scholar]
- 63.Greer D, Scripko P, Bartscher J, et al. Serial MRI changes in comatose cardiac arrest patients. Neurocrit Care. 2011;14:61–67. doi: 10.1007/s12028-010-9457-8. [DOI] [PubMed] [Google Scholar]
- 64.Greer DM. Mechanisms of injury in hypoxic-ischemic encephalopathy: implications to therapy. Semin Neurol. 2006;26:373–379. doi: 10.1055/s-2006-948317. [DOI] [PubMed] [Google Scholar]
- 65.Gray FD, Jr., Horner GJ. Survival following extreme hypoxemia. JAMA. 1970;211:1815–1817. [PubMed] [Google Scholar]
- 66.Schwamm LH, Koroshetz WJ, Sorensen AG, et al. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke. 1998;29:2268–2276. doi: 10.1161/01.str.29.11.2268. [DOI] [PubMed] [Google Scholar]
- 67.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 68.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Mlynash M, Campbell DM, Leproust EM, et al. Temporal and spatial profile of brain diffusion-weighted MRI after cardiac arrest. Stroke. 2010;41:1665–1672. doi: 10.1161/STROKEAHA.110.582452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinney HC, Korein J, Panigrahy A, et al. Neuropathological findings in the brain of Karen Ann Quinlan. The role of the thalamus in the persistent vegetative state. N Engl J Med. 1994;330:1469–1475. doi: 10.1056/NEJM199405263302101. [DOI] [PubMed] [Google Scholar]
- 71.Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327–1338. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- 72.Tong KA, Ashwal S, Holshouser BA, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56:36–50. doi: 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- 73.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melhem ER, Itoh R, Jones L, Barker PB. Diffusion tensor MR imaging of the brain: effect of diffusion weighting on trace and anisotropy measurements. AJNR Am J Neuroradiol. 2000;21:1813–1820. [PMC free article] [PubMed] [Google Scholar]
- 75.Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- 76.Marino S, Zei E, Battaglini M, et al. Acute metabolic brain changes following traumatic brain injury and their relevance to clinical severity and outcome. J Neurol Neurosurg Psychiatry. 2007;78:501–507. doi: 10.1136/jnnp.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The first two years of the patient’s recovery are documented longitudinally in pictures and videos that were shared by the patient and his family with their consent. All clinicians who appear in the video also provided consent. An interview with the patient was performed on day 783, at which time he reflected on the major milestones in his recovery.


