Abstract
Keratan sulfate (KS) comprises repeating disaccharides of galactose (Gal) and N-acetylglucosamine (GlcNAc). Residues of Gal and GlcNAc in KS are potentially modified with sulfate at their C-6 positions. The 5D4 monoclonal antibody recognizes KS structures containing Gal and GlcNAc, both 6-sulfated, and has been used most extensively to evaluate KS expression in mammalian brains. We previously showed that GlcNAc6ST1 is an enzyme responsible for the synthesis of the 5D4 epitope in developing brain and in the adult brain, where it is induced after injury. It has been unclear which sulfotransferase is responsible for Gal-6-sulfation within the 5D4 KS epitope in developing brains. We produced mice deficient in KSGal6ST, a Gal-6-sulfotransferase. Western blotting and immunoprecipitation revealed that all 5D4-immunoreactivity to proteins, including phosphacan, were abolished in KSGal6ST-deficient postnatal brains. Likewise, the 5D4 epitope, expressed primarily in the cortical marginal zone and subplate and dorsal thalamus, was eliminated in KSGal6ST-deficient mice. Disaccharide analysis showed the loss of Gal-6-sulfate in KS of the KSGal6ST-deficient brains. Transfection studies revealed that GlcNAc6ST1 and KSGal6ST cooperated in the expression of the 5D4 KS epitope in HeLa cells. These results indicate that KSGal6ST is essential for C-6 sulfation of Gal within KS in early postnatal brains.
Keywords: developing brain, extracellular matrix, glycosaminoglycan, keratan sulfate, phosphacan, sulfotransferase, 5D4
Introduction
Carbohydrate sulfation is a post-translational modification that occurs for molecules generally found on the cell surface and within the extracellular matrix. Sulfation modification is often altered in the brain in response to developing and pathological changes (Hosono-Fukao et al. 2012; Miyata et al. 2012). Examples of molecules with such modifications include glycosaminoglycans, selectin ligand mucins and sulfatides (Bishop et al. 2007; Eckhardt 2008; Nakato and Kimata 2002; Rosen 2004). Keratan sulfate (KS) is one of the glycosaminoglycans covalently attached to a core protein to form a keratan sulfate proteoglycan (KSPG). KS was first isolated from bovine cornea (Meyer et al. 1953). It has also been found in skeletal and nervous tissues (Funderburgh 2000). KS is classified into three different types—KS-I, KS-II and KS-III—based upon structural diversity in the oligosaccharides that link KS to the protein core (Funderburgh 2002). KS-I comprises N-linked KS chains that are abundant in the cornea. KS-II is composed of KS chains that are O-linked through N-acetylgalactosamine (GalNAc) and found in cartilage. The KS extended from O-linked mannose was described in proteoglycans extracted from the brain (Krusius et al. 1986) and defined later as KS-III (Funderburgh 2002). Intriguingly, up to 30% of all O-linked sugars in the brain are O-mannosyl-linked, although the structure is rare in most tissues (Chai et al. 1999; Krusius et al. 1986). Phosphacan is a major proteoglycan that carries KS-III in the central nervous tissue (Margolis et al. 1996).
KS consists of repeating disaccharide units of galactose (Gal) and N-acetylglucosamine (GlcNAc). The structure of KS chain-capping varies at their non-reducing termini, which include α(2-3)- or α(2-6)-linked sialic acid, α(1-3)-linked Gal and β(1-3)-linked sulfated GalNAc (Tai et al. 1996). Sulfation modifications, as well as elongation of the KS chains, are enzymatic. The vast majority of GlcNAc residues and a significant portion of adjacent Gal residues in KS are sulfated at their C6 positions. Keratan sulfate galactose 6-O-sulfotransferase (KSGal6ST, encoded by the gene CHST1) and chondroitin sulfotransferase-1 (C6ST-1) have been shown to generate C6-sulfated Gal in vitro (Fukuta et al. 1997; Habuchi et al. 1996; Torii et al. 2000). Genetic disruptions in KSGal6ST are associated with deficiency in the biosynthesis of KS in the eye (Patnode et al. 2013b); however, no gross morphological differences were observed in KSGal6ST-deficient mice (Patnode et al. 2013a; Patnode et al. 2013b). N-Acetylglucosamine 6-O-sulfotransferase-1 (GlcNAc6ST1, encoded by the gene CHST2), GlcNAc6ST2/HEC-GlcNAc6ST/LSST, GlcNAc6ST3/I-GlcNAc6ST, GlcNAc6ST4/C6ST-2 and GlcNAc6ST5/C-GlcNAc6ST (encoded by the gene CHST6) all can transfer a sulfate group to C6 of GlcNAc. Thus far, five members of the GlcNAc6ST family have been identified in humans, four of which have murine orthologs (Uchimura and Rosen 2006). Mutation in the CHST6 gene was identified as a cause of macular corneal dystrophy (Akama et al. 2000). GlcNAc6ST5 is essential for the synthesis of corneal KS. We have established that GlcNAc6ST1 and GlcNAc6ST2 impart L-selectin ligand activity to several mucins by elaborating sialyl 6-sulfo Lewis X, a C6-sulfated GlcNAc-containing oligosaccharide, and regulating lymphocyte homing to lymph nodes (Kawashima et al. 2005; Uchimura et al. 2005). We have also shown that GlcNAc6ST1 is an enzyme responsible for the synthesis of KS induced in the brain and spinal cord of adult mice after injury, and that loss of KS facilitates axonal regeneration/sprouting. (Ito et al. 2010; Zhang et al. 2006).
Sulfated patterns within glycosaminoglycans can be differentially detected with specific antibodies (Couchman et al. 1984; Dennissen et al. 2002; Fukui et al. 2002). Several monoclonal antibodies against KS have been developed; for example, 5D4, BCD4, TRA-1, I22, 373E1 and R10G (Andrews et al. 1984; Caterson et al. 1983; Funderburgh et al. 1990; Glant et al. 1986; Kawabe et al. 2013; Magro et al. 2003). Each of these antibodies recognizes sulfated epitopes within the KS chains. The monoclonal antibody 5D4, which was raised against KS-enriched proteoglycans of human articular cartilage (Caterson et al. 1983), has been used extensively to evaluate KS expression. Many studies have shown the susceptibility of 5D4 immunoreactivity to enzymatic treatment with keratanases. Although the linear pentasulfated hexasaccharide of poly-N-acetyllactosamine is sufficient for substantial inhibition of the binding of 5D4 to bovine corneal KS (Mehmet et al. 1986), the precise chemical structure of the 5D4 epitope is not completely understood. By immunocytochemistry, 5D4 reacts with cornea (Akama et al. 2000; Funderburgh et al. 1987), articular cartilage (Caterson et al. 1983; Poole et al. 1991) and N-linked KS in aggrecan (Poon et al. 2005). In the central nervous system (CNS), the epitope is constitutively expressed in a subpopulation of microglia of the adult brain (Bertolotto et al. 1998; Bertolotto et al. 1993; Jander et al. 2000). An increase in microglial expression of the epitope is also found in adult brains of animal models of bovine spongiform encephalopathy-like disease (Manuelidis et al. 1997), cerebral amyloid angiopathy (Miao et al. 2005) and familial Danish dementia (Vidal et al. 2009). The 5D4 epitope is also up-regulated in adult brain and spinal cord after injury (Imagama et al. 2011; Jones and Tuszynski 2002; Zhang et al. 2006).
In the developing brain, the expression of the 5D4 epitope is spatiotemporally regulated, as demonstrated in the rat (Meyer-Puttlitz et al. 1995; Miller et al. 1997). Using GlcNAc6ST1-deficient mice, we have previously shown that GlcNAc6ST1 is required for the expression of the 5D4 epitope in early postnatal mouse brains (Zhang et al. 2006). However, it has not been determined whether KSGal6ST is involved in synthesis of the 5D4 epitope in early postnatal mouse brain. In this study, we analyzed the expression of KSGal6ST, as well as the 5D4 epitope and GlcNAc6ST1, in the brains of postnatal day 1-21 mice. We found that the 5D4 reactivity in the cortex and thalamus of postnatal brains is eliminated in KSGal6ST-deficient mice, indicating that this enzyme is essential for the synthesis of the 5D4 epitope in developing postnatal mouse brains.
Materials & Methods
Antibodies
The following materials were obtained commercially from the indicated sources. The 5D4 anti-KS antibody and the 6B4 mouse monoclonal anti-phosphacan antibody were from Seikagaku Corporation (Tokyo, Japan); goat anti-KSGal6ST (CHST1) antibody was from Santa Cruz Biotechnology, Inc. (Dallas, TX); rabbit anti-GlcNAc6ST-1 (CHST2) polyclonal antibody and anti-actin antibody (clone AC-40) were from Sigma-Aldrich (St. Louis, MO); horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG1 was from Caltag (Burlingame, CA); HRP-conjugated goat anti-rabbit IgG (H+L) was from Cell Signaling Technology (Danvers, MA); and HRP-conjugated goat anti-mouse IgG2b, HRP-conjugated goat anti-mouse IgG (H+L) and Cy™3-conjugated goat anti-mouse IgG1 were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Animals
C57BL/6J mice (6 weeks old) were purchased from SLC Inc. (Hamamatsu, Japan). The generation of KSGal6ST-deficient mice (Patnode et al. 2013) and GlcNAc6ST1-deficient (Uchimura et al. 2004) mice was as previously described. GlcNAc6ST1-deficient mice have no apparent gross abnormalities (Uchimura et al. 2004). All mice were maintained in the animal facilities of Nagoya University Graduate School of Medicine and the National Center for Geriatrics and Gerontology (NCGG). All experiments were approved by the Animal Research Committee of Nagoya University and NCGG, and performed in accordance with the guidelines of Nagoya University and NCGG.
Northern Blot Analysis
Total RNA was isolated from C57BL/6J mouse tissues using TRIzol (Invitrogen; Carlsbad, CA) following the manufacturer’s instructions. The radioactive probes for mRNA detection of KSGal6ST (Fukuta et al. 1997) and GlcNAc6ST1, and mouse multiple tissue Northern membranes were prepared as described previously (Uchimura et al. 1998a). Human Multiple Tissue Northern Blots were purchased from Clontech Laboratories, Inc. (Mountain View, CA). Hybridization was performed as described previously (Uchimura et al. 1998b). The radioactivity on the membrane was determined with a BAS 2000 bio-imaging analyzer (Fuji Film; Tokyo, Japan). Hybridization with a cDNA probe of glyceraldehyde 3-phosphate dehydrogenase to test for equal RNA loading was as previously described (Uchimura et al. 1998a; Uchimura et al. 1998b).
Recombinant Proteins
Plasmids harboring cDNAs of mouse KSGal6ST (pCMV6-Entry Myc/DDK-mChst1) and GlcNAc6ST-1 (pCMV6-mChst2) were purchased from OriGene Technologies, Inc. (Rockville, MD). HeLa cells were transfected with the plasmids by using FuGENE 6 (Promega; Fitchburg, WI) as per the manufacturer’s instructions. Forty-eight hr later, the cells were washed with phosphate-buffered saline (PBS) supplemented with Complete protease inhibitor cocktail (Roche Applied Science; Mannheim, Germany), and then collected in PBS in a tube. The cell suspension was subjected to three repeated cycles of freezing and thawing. After centrifugation at 12,000 × g for 10 min, the pellet was suspended in 10 mM Tris-HCl containing 1 mM EDTA (pH 8.0) and 1% Triton X-100, followed by mild agitation at 4C for 15 min. The supernatant obtained by centrifugation at 12,000 × g for 10 min was used as the “membrane fraction”. HeLa cells do not express transcripts for either KSGal6ST (Figure 6D) or GlcNAc6ST1 (Uchimura et al. 1998b).
Figure 6.
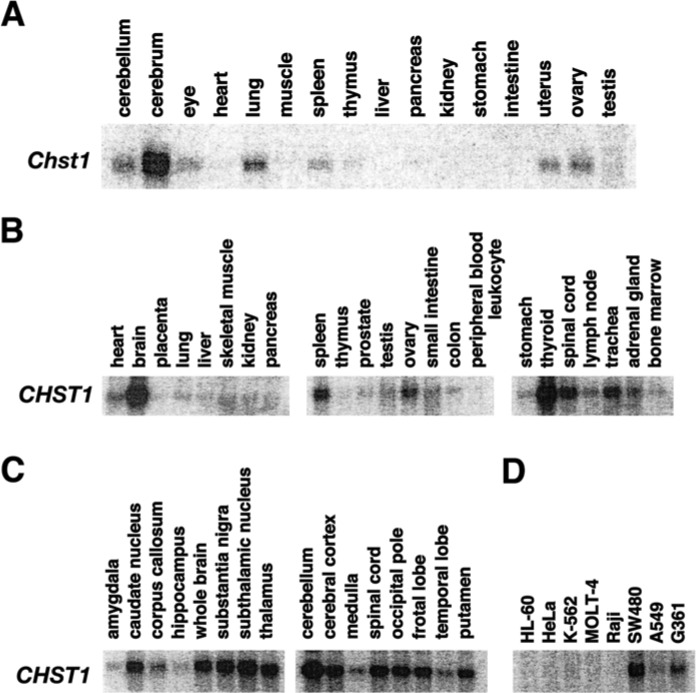
Northern blot analyses of mouse Chst1 and human CHST1 in adult tissues. (A) KSGal6ST mRNA (Chst1) expression in adult mouse tissues. Each lane contained 20 µg of total RNA. (B–D) KSGal6ST mRNA (CHST1) expression in various human tissues (B), sub-regions of the brain (C) and various tumor cells (D). Each lane contained 2 µg of poly (A)+ RNA.
Western Blot Analysis and Immunoprecipitation
Fractionation of brain samples was performed as described previously (Hosono-Fukao et al. 2012). Snap-frozen mouse cortices (~25 mg) from postnatal and adult mice were fragmented by sonicating in cold Tris-buffered saline (TBS) containing Complete protease inhibitor. The material was ultracentrifuged at 100,000 × g for 20 min at 4C. The supernatant was used as the “TBS-soluble fraction”. The pellet was re-suspended in TBS containing 1% SDS and Complete protease inhibitor. The suspension was centrifuged at 12,000 rpm for 20 min at room temperature. The supernatant was used as the “SDS-soluble fraction”. Forty micrograms of proteins in the TBS-soluble fractions (for 5D4 epitope detection) and 20 µg of proteins in the SDS-soluble fractions (for KSGal6ST and GlcNAc6ST1 detection) were subjected to SDS-PAGE on NuPAGE 3% to 8% gels (Invitrogen) and on 7.5% gels (Wako; Osaka, Japan), respectively. Non-reducing SDS-PAGE was carried out to detect KSGal6ST and GlcNAc6ST1 proteins. To determine the effects of glycosaminoglycan-degrading enzymes, 1% Triton-soluble whole-tissue lysates were prepared and used as the “Triton-soluble fractions”. The samples were treated with enzymes at 37C overnight before undergoing electrophoresis. The enzymes used were a mix of 1 mU heparinase I (Sigma), 0.25 mU heparinase II (Sigma) and 0.1 mU heparinase III (Sigma), 50 mU chondroitinase ABC (Seikagaku), 0.5 mU keratanase II (Seikagaku) or 250 mTRU hyaluronidase (Seikagaku). For pretreatment with a mix of heparitinases, MgCl2 (10 mM final) was added to the reaction mixture. Electrophoresed samples were blotted onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA). Blots were blocked with TBS containing 0.1% Tween-20 (TBS-T) and 5% skim milk (BD Biosciences; Franklin Lakes, NJ) for 1 hr and then incubated with primary antibody: anti-KS antibody (5D4; 1:500 dilution), anti-KSGal6ST antibody (A-2; 1:1000 dilution), anti-GlcNAc6ST-1 antibody (1:1000 dilution), in 5% skim milk/TBS-T overnight at 4C, or anti-actin antibody (1:2500 dilution) for 1 hr at room temperature. Membranes were washed with TBS-T and incubated for 1 hr with horseradish peroxidase (HRP)-conjugated secondary antibody. Bound antibodies were detected with Super Signal West Pico Chemiluminescent reagent (Thermo Scientific; Wilmington, DE). For immunoprecipitation, Triton X-100 was added to 200 µg of proteins in the “TBS-soluble fraction” of the cortex to a final concentration of 1% (w/v). The suspension was heated for 10 min at 95C and then subjected to the digestion with chondroitinase ABC. The digested materials were mixed with the 6B4 rabbit polyclonal anti-phosphacan antibody (a generous gift from N. Maeda) for 2 hr at 4C. The immune complex was precipitated with 30 μL of a 50% (v/v) suspension of protein A sepharose for 1 hr at 4C and then washed three times with PBS containing 0.3% Triton X-100. The immunocomplexes bound to the protein A beads were isolated by centrifugation and then subjected to immunoblotting and lectin blot analyses with biotinylated Lycopersicon esculentum lectin (LEL) (5 µg/ml; Vector Laboratories, Burlingame, CA) and HRP-conjugated streptavidin (1:10,000 dilution; Caltag).
Preparation and Structural Analysis of Brain Keratan Sulfate
Brain KS was isolated and analyzed as described previously for heparan sulfate (Hosono-Fukao et al. 2012) with slight modifications. Mouse brains were dissected from postnatal day 1 mice (~150 mg), suspended in 2 ml of 0.2 N NaOH, and then incubated overnight at room temperature. Samples were neutralized with 4 N HCl and then treated with DNase I and RNase A (0.04 mg/ml each) (Roche Applied Science) in 50 mM Tris-HCl, pH 8.0, 10 mM MgCl2 for 3 hr at 37C. Subsequently, the samples were treated with actinase E (0.08 mg/ml) (Kaken Pharmaceutical Co., Ltd., Tokyo, Japan) overnight at 37C. Samples were heated to inactivate enzymes and then centrifuged at 5000 × g at 4C for 10 min. The supernatant was collected, mixed with an equal volume of 50 mM Tris-HCl, pH 7.2, and then applied to a diethylaminoethanol (DEAE) Sepharose column for chromatography. The column was washed with 50 mM Tris-HCl, pH 7.2, and 0.1 M NaCl. KS bound to the column was eluted with 50 mM Tris-HCl, pH 7.2, and 2 M NaCl and then precipitated with ethanol. KS was pre-treated with a mix of 50 mU neuraminidase (Arthrobacter ureafaciens: Nacalai Tesque, Kyoto, Japan) and 2 µU α1,3/4-L-fucosidase (Streptomyces sp. 142: Takara Bio Inc., Shiga, Japan) for 2 hr at 37C. KS was precipitated using ethanol and then digested with 0.5 mU keratanase II (Bacillus sp. Ks 36: Seikagaku, Tokyo, Japan) at 37C overnight. The oligosaccharide compositions of KS were determined by reversed-phase ion-pair chromatography with post-column fluorescent labeling (Patnode et al. 2013). The level of KS content was determined by summing the amounts of Galß1-4GlcNAc(6S) and Gal(6S)ß1-4GlcNAc(6S) disaccharides detected in each sample. Galß1-4GlcNAc(6S) and Gal(6S)ß1-4GlcNAc(6S) were provided by Nobuo Sugiura, Aichi Medical University, and used as authentic standards.
Immunohistochemistry
Fresh brains from developing and adult wild type (WT), KSGal6ST KO and GlcNAc6ST-1 KO mice were embedded in the O.C.T. compound (Sakura Finetek, Torrance, CA) and then frozen using liquid nitrogen. Cryostat-cut sections (10 μm thick) were prepared on MAS-coated glass slides (SF17293, Matsunami, Osaka, Japan), fixed with ice-cold acetone for 15 min, and then air-dried for 30 min. Sections were incubated with 3% BSA in PBS for 15 min at room temperature for blocking and then incubated with 5D4 anti-KS antibody (1:80 dilution) overnight at 4C. Sections were incubated with Cy3-anti-mouse IgG1 (1:250 dilution) for 30 min at room temperature. After washing with PBS, sections were incubated with Hoechst 33342 solution (Dojindo, Japan; 1:1000 dilution) for 5 min at room temperature for staining nuclei. Stained sections were mounted in FluorSave Reagent (Merck). Digital images were captured by fluorescent microscopy (model BX50, Olympus) at the same setting for all images.
Immunocytochemistry
HeLa cells that were transfected with pCMV6 Entry Myc/DDK-mChst1 and/or pCMV6-mChst2 as described above, and were grown on culture slides (BD Falcon). Forty-eight hr later, the cells were washed with PBS and then fixed with 4% paraformaldehyde in PBS for 15 min at room temperature. Sections were incubated with 3% BSA in PBS for 15 min at room temperature for blocking and then incubated with 5D4 anti-KS antibody (1:500 dilution) for 1 hr at room temperature. Bound antibody was detected with Cy3-anti-mouse IgG1 antibody (1:250 dilution). The transfection efficiency was measured by counting the percentage of green-fluorescent cells in parallel experiments with an expression plasmid encoding green fluorescent protein (GFP). The efficiency was determined to be ~70%.
Statistical Analysis
All data are presented as the mean ± S.D. unless noted otherwise. The values were analyzed by one-way ANOVA with Tukey’s test using Prism software (GraphPad Software, La Jolla, CA). P values less than 0.05 were considered to be statistically significant.
Results
Expression of mRNAs and Proteins of KSGal6ST and GlcNAc6ST1 in Early Postnatal Brains
mRNA expression of KSGal6ST/Chst1 and GlcNAc6ST1/Chst2 in mouse brains at postnatal days (P) 1 to P28 was examined by Northern blotting analysis. Transcripts for Chst1 (2.8 kb) and Chst2 (3.9 kb) were detected in the cortex and cerebellum throughout the postnatal period. mRNA levels of these genes increased from P7 to P28 (Fig. 1A). We then investigated whether KSGal6ST and GlcNAc6ST1 proteins were expressed in the cortex at different developmental stages. We carried out western blotting using specific antibodies: mouse anti-KSGal6ST and rabbit anti-GlcNAc6ST1. We confirmed the specificity of these antibodies using recombinant proteins in “membrane fractions” from HeLa cells transfected with pCMV6-Entry Myc/DDK-mChst1 or pCMV6-mChst2 (Fig. 1B). Anti-KSGal6ST and anti-GlcNAc6ST1 antibodies specifically reacted with recombinant KSGal6ST (120 kDa) and GlcNAc6ST1 proteins (135 kDa), respectively, under non-reducing conditions (Fig. 1B). For further validation of these antibodies, we employed mice deficient in the two enzymes. Anti-KSGal6ST and anti-GlcNAc6ST1 antibodies detected bands at 120 kDa and 135 kDa, respectively, in “membrane fractions” of P1 WT cortex (Fig. 1C). Bands with corresponding sizes were not seen in their gene knock-out (KO) mice (Fig. 1C). We prepared “SDS-soluble fractions” of the cortex from postnatal days 1, 7, 14, and 21 and adult wild-type mice. These samples were subjected to western blotting analysis, and we observed expression of KSGal6ST in the P1-21 and adult cortex samples. Higher levels of protein expression were seen at P7, 14 and 21 (Fig. 1D). The highest level of GlcNAc6ST1 expression was observed at P1, with expression decreasing with age (Fig. 1D). Interestingly, the size of the immunoreactive band seen in P1 gradually increased with age to 145 kDa at P21.
Figure 1.
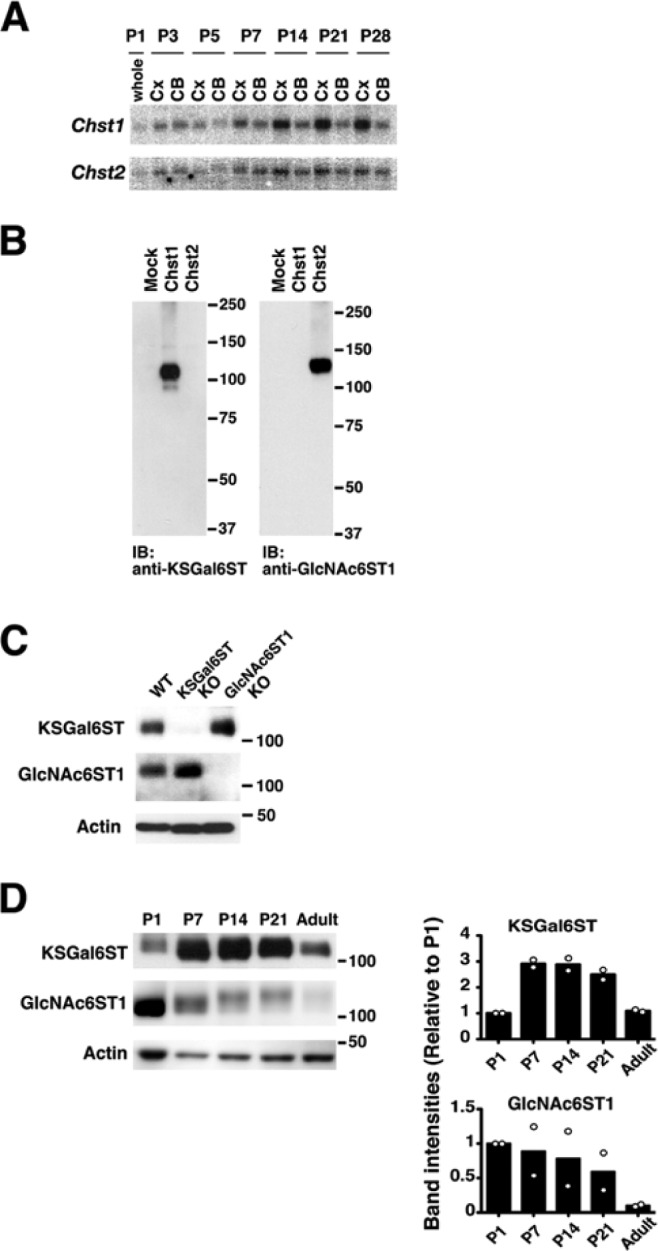
mRNA and protein expression of KSGal6ST and GlcNAc6ST1 in early postnatal brains. (A) KSGal6ST (Chst1) and GlcNAc6ST1 (Chst2) mRNA expression in the cortex (Cx) and cerebellum (CB) at postnatal days (P) 1, 3, 5, 7, 14, 21 and 28. Each lane contained 10 µg of total RNA. (B–D) Immunoblot analysis with anti-KSGal6ST and anti-GlcNAc6ST1 antibodies, performed as described in Materials & Methods. “Membrane fractions” were prepared from HeLa cells that were transfected with an empty vector (Mock), plasmid harboring cDNA of mouse KSGal6ST (Chst1) or mouse GlcNAc6ST1 (Chst2) (B), and from cortices of postnatal day 1 WT, KSGal6ST KO and GlcNAc6ST1 KO mice (C). KSGal6ST and GlcNAc6ST1 expression in “SDS-soluble fractions” prepared from postnatal day (P) 1, 7, 14, 21, and adult WT mouse cortices. Intensities of bands relative to P1 were measured by densitometry. Open dots represent data from two independent determinations. (D) Columns show means of these data points.
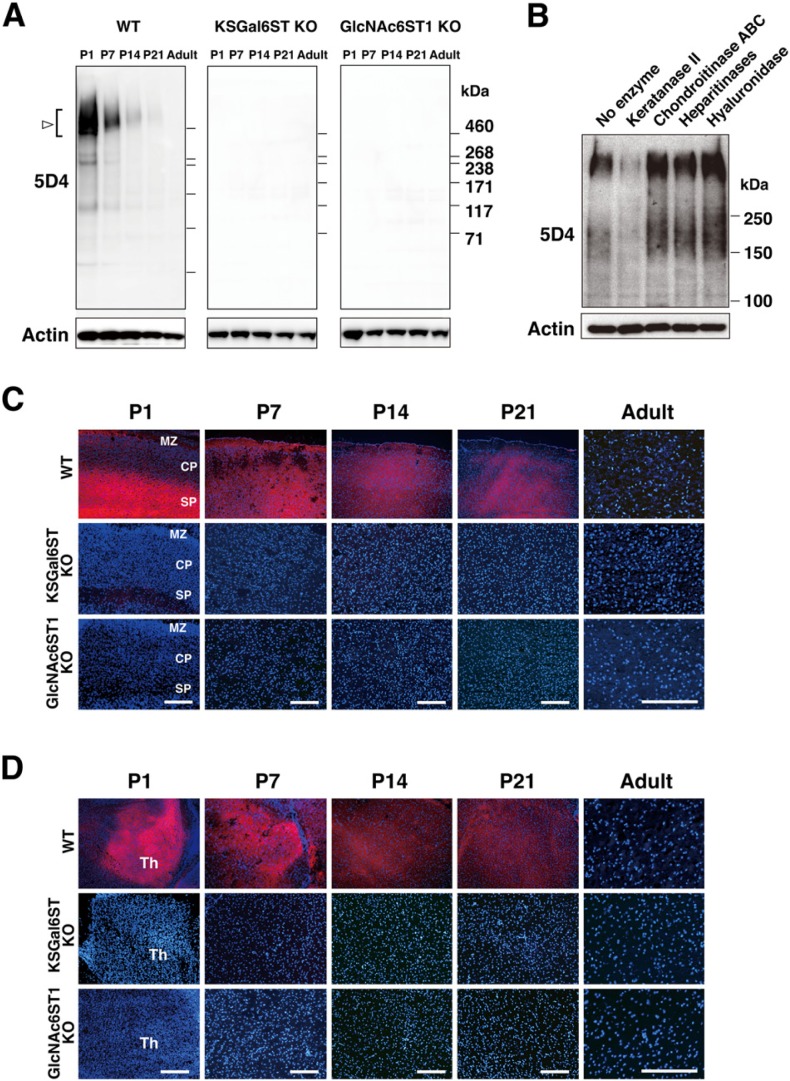
Deficient Expression of the 5D4 KS Epitope in Early Postnatal Brains of KSGal6ST KO Mice
We carried out 5D4 western blot analysis on “TBS-soluble fractions” obtained from the cortex of mouse postnatal brains. A major smear band was detected at approximately 500 kDa (Fig. 2A). Minor bands were present at 280, 240 and 150 kDa in the P1 cortex (Fig. 2A). The expression level of the 5D4 epitope in these bands was the highest in P1. 5D4 reactivity to these bands declined with postnatal time. Expression of the 5D4 epitope was totally absent in postnatal brain samples from KSGal6ST KO and GlcNAc6ST1 KO mice (Fig. 2A). To test if the 5D4 epitope is within KS chains in postnatal brains, “Triton-soluble fractions” were prepared from tissue homogenates of P1 cortex and then incubated with different glycosaminoglycan-degrading enzymes. Pre-digestion with keratanase II greatly reduced the 5D4 immunoreactivity (Fig. 2B). Chondroitinase ABC, heparitinases (a mixture of heparinase I, heparinase II and heparinase III) and hyaluronidase did not affect reactivity, while these enzymes produced a slight increase in reactivity (Fig. 2B). Robust expression of the 5D4 epitope in the subplate and marginal zone of P1 cortex was observed, as previously described for rat tissue (Miller et al. 1997) (Fig. 2C). These staining signals were primarily seen in extracellular spaces. The expression became broad in all layers of the cortex from P7 to P21. The 5D4 immunoreactivity was abolished in the postnatal brains of KSGal6ST KO and GlcNAc6ST1 KO mice (Fig. 2C). Expression of the 5D4 epitope in the dorsal thalamus of postnatal brains was also eliminated in KSGal6ST KO and GlcNAc6ST-1 KO mice (Fig. 2D). 5D4 immunoreactivity detected in cells in the fimbriae of the hippocampus and the medulla of the adult cerebellum was also absent in the KO mice (data not shown).
Figure 2.
Expression of the 5D4 keratan sulfate epitope in postnatal brains and its loss in mice deficient in KSGal6ST and GlcNAc6ST1. (A) Expression of the 5D4 keratan sulfate epitope in “TBS-soluble fractions” prepared from P1-adult wild type (WT), KSGal6ST KO and GlcNAc6ST1 KO cortices (open arrow/bracket). (B) Effects of glycosaminoglycan-degrading enzymes on the 5D4 epitope in P1 WT cortex. (C–D) The 5D4 epitope (red) expression in P1-adult WT, KSGal6ST KO and GlcNAc6ST1 KO cortices (C) and thalami (D). MZ, marginal zone; CP, cortical plate; SP, subplate; Th, thalamus. Bars: 120 µm.
Diminished KS Synthesis in Early Postnatal Brains of KSGal6ST KO Mice
To see if KS synthesis is altered in the early postnatal brain of the sulfotransferase KO mice, the P1 cortices of mice deficient in KSGal6ST and GlcNAc6ST1 were subjected to reversed-phase ion-pair high-performance liquid chromatography. The disaccharide compositions of the KS were determined. We found that the amount of a mono-sulfated disaccharide, Galβ1-4GlcNAc(6S), was not altered in KSGal6ST KO but decreased in GlcNAc6ST-1KO to 25% of the WT level (Fig. 3A). The amount of the di-sulfated disaccharide, Gal(6S)β1-4GlcNAc(6S), was reduced to an undetectable level in both KSGal6ST KO and GlcNAc6ST1 KO mice (Fig. 3A). The KS content in the tissue was reduced to 41% and 9% of the WT level in KSGal6ST KO and GlcNAc6ST1 KO, respectively (Fig. 3B).
Figure 3.
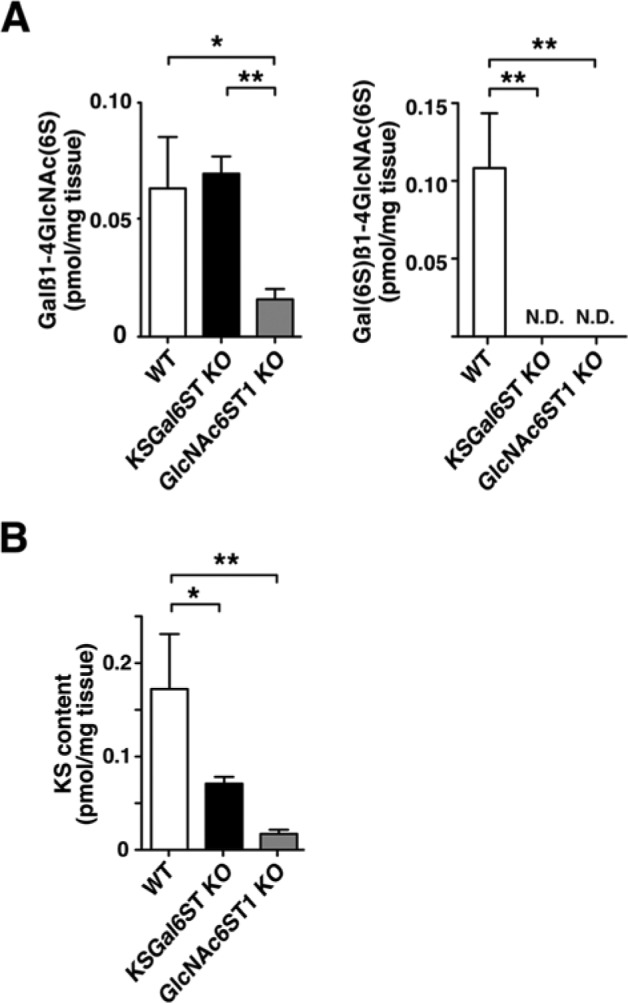
Disaccharide analysis of keratan sulfate in postnatal day 1 mouse brain. (A–B) Keratan sulfate disaccharide compositions and the content of keratan sulfate in postnatal day 1 wild type (WT) (n=3), KSGal6ST KO (n=3) and GlcNAc6ST1 KO brains (n=3). The level of keratan sulfate content was determined by summing the amounts of Galβ1-4GlcNAc(6S) and Gal(6S)β1-4GlcNAc(6S) in each sample. *, p<0.05; **, p<0.001; ND, not detectable.
Elaboration of the 5D4 Epitope in HeLa Cells by Co-transfecting cDNAs of KSGal6ST and GlcNAc6ST1
We then investigated whether KSGal6ST and GlcNAc6ST1 are sufficient to elaborate the 5D4 epitope in culture cells. HeLa cells were transfected with pcDNA3.1, pCMV6-Entry Myc/DDK-mChst1, pCMV6-mChst2 or both pCMV6-Entry Myc/DDK-mChst1 and pCMV6-mChst2 (Fig. 4). Immunostaining by 5D4 showed that 3.2 ± 1.2% of HeLa cells transfected with cDNAs for both KSGal6ST and GlcNAc6ST1 were positive for the stained signals. Cells transfected with either cDNA alone did not show expression of the 5D4 epitope.
Figure 4.
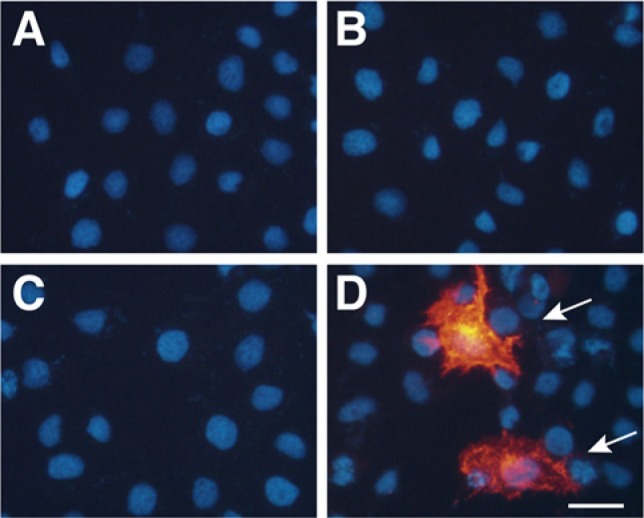
Expression of the 5D4 keratan sulfate epitope in transfected HeLa cells. Immunocytochemistry of HeLa cells that were transfected with (A) empty vector, (B) pCMV6-Entry Myc/DDK-mChst1, (C) pCMV6-mChst2, or (D) both pCMV6-Entry Myc/DDK-mChst1 and pCMV6-mChst2. The cells were stained with the 5D4 antibody (red, indicated by the arrow in D) and Hoechst (blue). Bar: 30 µm.
Loss of the 5D4 Epitope in Phosphacan Expressed in P1 Brains of KSGal6ST KO Mice
Phosphacan is a KSPG in nervous tissue (Margolis et al. 1996). Both the 6B4 rabbit polyclonal and the 6B4 mouse monoclonal antibodies recognize this proteoglycan (Maeda et al. 1995). A rabbit 6B4 immunoprecipitate of TBS-soluble fraction of WT P1 brains reacted with 5D4 by western blotting. This reactivity was absent in corresponding 6B4 immunoprecipitates from KSGal6ST KO and GlcNAc6ST1 KO mice (Fig. 5). The 6B4 mouse monoclonal antibody and LEL, a lectin that binds to polylactosamine (Togayachi et al. 2007), recognized bands in 6B4 immunoprecipitate samples of all three genotypes (Fig. 5). The reactivity of LEL was slightly increased in the 6B4 immunoprecipitate of GlcNAc6ST1 KO brain as compared with that from WT samples (Fig. 5).
Figure 5.
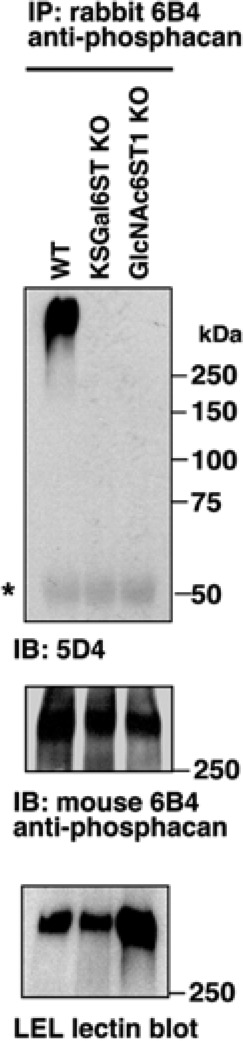
Loss of the 5D4 epitope in phosphacan expressed in postnatal day 1 brain of mice deficient in KSGal6ST and GlcNAc6ST1. “TBS-soluble fractions” prepared from P1 wild type (WT), KSGal6ST KO and GlcNAc6ST1 KO cortices were treated with chondroitinase ABC and then precipitated with the 6B4 rabbit polyclonal anti-phosphacan antibody. Immunoprecipitated materials were blotted with 5D4 or the 6B4 mouse monoclonal anti-phosphacan antibody. Lectin blot analysis for the materials was performed with LEL, which binds to polylactosamines (Togayachi et al. 2007). The asterisk indicates the position of the immunoglobulin heavy chain.
mRNA Expression of KSGal6ST/CHST1 in Adult Tissues
In adult, mRNA levels of mouse Chst1 and human CHST1 were the highest in central nervous system tissues(Figs. 6A, 6B). Human CHST1 mRNA was broadly expressed in subregions of the brain tested (Fig. 6C). High and moderate expression of the CHST1 gene in SW480 and G361 cells were observed, respectively (Fig. 6D).
Discussion
In the present study, we demonstrate that the 5D4 KS epitope is strongly expressed in mouse postnatal brains, especially in the cortex and thalamus, and that the level of the epitope declined with age. Immunohistochemistry and western blot analysis showed that the 5D4 epitope was absent in mice deficient in either KSGal6ST or GlcNAc6ST1. These results indicate that KSGal6ST and GlcNAc6ST1 are essential for elaboration of the 5D4 epitope in brain at an early postnatal stage. Our structural analysis revealed that KSGal6ST is essential for Gal-6-sulfation of Gal(6S)β1-4GlcNAc(6S) within KS, but not for GlcNAc-6-sulfation of Galβ1-4GlcNAc(6S) in P1 mouse brains. In GlcNAc6ST1 KO brains, Galβ1-4GlcNAc(6S) was substantially reduced. The residual amount of Galβ1-4GlcNAc(6S) could be attributed to activities of other GlcNAc6STs. Gal(6S)β1-4GlcNAc(6S) was at an undetectable level in the GlcNAc6ST1 KO brains. This may be explained by the fact that GlcNAc-6-sulfation occurs only on GlcNAc residues on the non-reducing termini of nascent carbohydrate chains (Akama et al. 2002; Uchimura et al. 1998a), and that Gal-6-sulfation requires GlcNAc-6-sulfation. Analysis of mice lacking other members of Gal6ST and GlcNAc6ST would be one avenue to address these issues. Why parallel increases in the amount of Galβ1-4GlcNAc(6S) in KSGal6ST KO brains was not observed and certain amounts of Gal(6S)β1-4GlcNAc(6S) were not detected in GlcNAc6ST1 KO brains remain unexplained. Our procedures for the quantification and analysis of disaccharide compositions of KS include enzymatic cleavage and separation of digestion products by reversed-phase ion-pair chromatography, thus relying upon substrate specificity of keratanase II. The enzyme hydrolyses the β-1,3-glucosaminidic linkage to Gal in KS. Keratanase II requires the sulfate at the C6 position of the participating glucosamine. If the non-sulfated disaccharide, N-acetyllactosamine, exists on the non-reducing side of Galβ1-4GlcNAc(6S) and Gal(6S)β1-4GlcNAc(6S), these sulfated disaccharides are not released by digestion with keratanase II. Some of the sulfated disaccharides could be underestimated. The subtle increase in LEL recognition in the GlcNAc6ST1 KO sample could be due to altered synthesis of N-acetyllactosamine. Alternative approaches, such as FACE (Plaas et al. 2001) and NMR (Brown et al. 1994), could provide insight to explain why the deficiency in GlcNAc6ST1 can cause incremental changes in the proportion of the non-sulfated disaccharides within KS and/or an increase in the elongation of polylactosamine in a distinct branch of the glycan. It is of note that 6-sulfation of Gal by KSGal6ST competes with fucosylation of 6-sulfated GlcNAc within lactosamine (Hiraoka et al. 2007). We thus cannot rule out the possible involvement of fucosyltransferases in the synthesis of cerebral KS at an early postnatal stage.
Our transfection experiments revealed that both KSGal6ST and GlcNAc6ST1 are sufficient to reconstitute the 5D4 epitope in HeLa cells. This result is consistent with a previous report that KS oligosaccharides comprising both 6-sulfated Gal and 6-sulfated GlcNAc residues markedly inhibit the 5D4 binding to KS (Mehmet et al. 1986). In accordance with a previous report showing that human GlcNAc6ST5/CHST6 and mouse GlcNAc6ST3/Chst5 cooperate with KSGal6ST to produce the 5D4 epitope in HeLa cells (Akama et al. 2001), mouse GlcNAc6ST1 cooperates with KSGal6ST to produce this epitope. Gal-6-sulfation by KSGal6ST should occur following GlcNAc-6-sulfation by GlcNAc6ST1. This could be explained by a previous report stating that KSGal6ST preferentially transfers sulfate to a Gal residue adjacent to 6-sulfated GlcNAc (Torii et al. 2000).
KSPGs have been identified and characterized in the CNS (Funderburgh 2000). Among these, phosphacan, a secreted variant of protein-tyrosine phosphatase receptor type Z (Ptprz)/protein tyrosine phosphatase ζ (PTP ζ )/receptor protein tyrosine phosphatase β (RPTPβ), is expressed in early postnatal brain of rodents (Margolis et al. 1996; Miller et al. 1997). Monoclonal anti-DSD-1 antibody, 473HD, recognizes phosphacan (Faissner et al. 1994) and stains the marginal zone and subplate of the developing mouse brain (Dwyer et al. 2012). We have previously shown that materials of mouse P1 brains precipitated with an anti-phosphacan antibody were immunoreactive with 5D4 (Zhang et al. 2006). Indeed, immunoprecipitation experiments revealed that the 5D4 epitope in phosphacan is totally abolished in P1 brains of mice deficient in either KSGal6ST or GlcNAc6ST1. Although there is the possibility that the 5D4-positive molecules in P1 mouse cortex include aggrecan (Funderburgh 2000) and proteolytically released ectodomains of Ptprz (Chow et al. 2008), it is most likely that the major 5D4-positive band, with a size above 460 kDa in P1 mouse cortex, is phosphacan. Phosphacan is a chondroitin sulfate proteoglycan that is also modified with KS and binds to tenascin but not to laminin or fibronectin (Grumet et al. 1994). During the early postnatal stage, growth of neurites and synapse formation actively proceed in the cortex. Phosphacan inhibits cell adhesion to tenascin (Grumet et al. 1994) and promotes neurite extension of cortical neurons (Maeda et al. 1995). It has been proposed that these phosphacan/RPTPβ cell-matrix and cell-cell interactions in the developing brain might be important for cortical histogenesis. RPTPβ signaling is regulated by its O-mannosyl glycan structures (Abbottet al. 2008). It is plausible that KS modification on the O-mannosyl-linked glycans could be involved in signaling mediated by phosphacan/RPTPβ in early postnatal brains.
In conclusion, we found that KSGal6ST is essential for elaboration of 6-sulfation of Gal and the 5D4 epitope within KS in postnatal mouse brains. These observations corroborate with our previous finding that GlcNAc6ST1 is essential for this epitope. The possible involvement of the KS epitope and its underlying sulfotransferases in the developing postnatal cortex, as well as injury and disease settings, are accessible to further investigation by employing gene-knockout mice.
Acknowledgments
We thank Steven D. Rosen for providing KSGal6ST KO mice and critical reading of the manuscript. We also thank Michael L. Patnode, Tomomi Hosono-Fukao and Kuniko Takanose for assistance, Nobuo Sugiura for providing keratan sulfate oligosaccharides, and Nobuaki Maeda for the generous gift of the 6B4 antibody.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Japanese Health and Labour Sciences Research Grants [Comprehensive Research on Aging and Health H19-001 and H22-007 to K.U.], Grants-in-Aid from the Ministry of Education, Science, Sports and Culture [22790303 and 24590349 to K.U., 23790426 to H.H., and Scientific Research on Innovative Areas to K.U. and K.K.] and in part by the Takeda Science Foundation [to K.U.].
References
- Abbott KL, Matthews RT, Pierce M. (2008). Receptor tyrosine phosphatase beta (RPTP beta) activity and signaling are attenuated by glycosylation and sbsequent cell surface galectin-1 binding. J Biol Chem 283:33026-33035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama TO, Misra AK, Hindsgaul O, Fukuda MN. (2002). Enzymatic synthesis in vitro of the disulfated disaccharide unit of corneal keratan sulfate. J Biol Chem 277:42505-42513 [DOI] [PubMed] [Google Scholar]
- Akama TO, Nakayama J, Nishida K, Hiraoka N, Suzuki M, McAuliffe J, Hindsgaul O, Fukuda M, Fukuda MN. (2001). Human corneal GlcNac 6-O-sulfotransferase and mouse intestinal GlcNac 6-O-sulfotransferase both produce keratan sulfate. J Biol Chem 276:16271-16278 [DOI] [PubMed] [Google Scholar]
- Akama TO, Nishida K, Nakayama J, Watanabe H, Ozaki K, Nakamura T, Dota A, Kawasaki S, Inoue Y, Maeda N, Yamamoto S, Fujiwara T, Thonar EJMA, Shimomura Y, Kinoshita S, Tanigami A, Fukuda MN. (2000). Macular corneal dystrophy type I and type II are caused by distinct mutations in a new sulphotransferase gene. Nat Genet 26:237-241 [DOI] [PubMed] [Google Scholar]
- Andrews PW, Banting G, Damjanov I, Arnaud D, Avner P. (1984). Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma 3:347-361 [DOI] [PubMed] [Google Scholar]
- Bertolotto A, Agresti C, Castello A, Manzardo E, Riccio A. (1998). 5D4 keratan sulfate epitope identifies a subset of ramified microglia in normal central nervous system parenchyma. J Neuroimmunol 85:69-77 [DOI] [PubMed] [Google Scholar]
- Bertolotto A, Caterson B, Canavese G, Migheli A, Schiffer D. (1993). Monoclonal antibodies to keratan sulfate immunolocalize ramified microglia in paraffin and cryostat sections of rat brain. J Histochem Cytochem 41:481-487 [DOI] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. (2007). Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446:1030-1037 [DOI] [PubMed] [Google Scholar]
- Brown GM, Huckerby TN, Nieduszynski IA. (1994). Oligosaccharides derived by keratanase II digestion of bovine articular cartilage keratan sulphates. Eur J Biochem 224:281-308 [DOI] [PubMed] [Google Scholar]
- Caterson B, Christner JE, Baker JR. (1983). Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem 258:8848-8854 [PubMed] [Google Scholar]
- Chai W, Yuen CT, Kogelberg H, Carruthers RA, Margolis RU, Feizi T, Lawson AM. (1999). High prevalence of 2-mono- and 2,6-di-substituted manol-terminating sequences among O-glycans released from brain glycopeptides by reductive alkaline hydrolysis. Eur J Biochem 263:879-888 [DOI] [PubMed] [Google Scholar]
- Chow JP, Fujikawa A, Shimizu H, Suzuki R, Noda M. (2008). Metalloproteinase- and gamma-secretase-mediated cleavage of protein-tyrosine phosphatase receptor type Z. J Biol Chem 283:30879-30889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR, Caterson B, Christner JE, Baker JR. (1984). Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307:650-652 [DOI] [PubMed] [Google Scholar]
- Dennissen MA, Jenniskens GJ, Pieffers M, Versteeg EM, Petitou M, Veerkamp JH, van Kuppevelt TH. (2002). Large, tissue-regulated domain diversity of heparan sulfates demonstrated by phage display antibodies. J Biol Chem 277:10982-10986 [DOI] [PubMed] [Google Scholar]
- Dwyer CA, Baker E, Hu H, Matthews RT. (2012). RPTPzeta/phosphacan is abnormally glycosylated in a model of muscle-eye-brain disease lacking functional POMGnT1. Neuroscience 220:47-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt M. (2008). The role and metabolism of sulfatide in the nervous system. Mol Neurobiol 37:93-103 [DOI] [PubMed] [Google Scholar]
- Faissner A, Clement A, Lochter A, Streit A, Mandl C, Schachner M. (1994). Isolation of a neural chondroitin sulfate proteoglycan with neurite outgrowth promoting properties. J Cell Biol 126:783-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Feizi T, Galustian C, Lawson AM, Chai W. (2002). Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat Biotechnol 20:1011-1017 [DOI] [PubMed] [Google Scholar]
- Fukuta M, Inazawa J, Torii T, Tsuzuki K, Shimada E, Habuchi O. (1997). Molecular cloning and characterization of human keratan sulfate Gal-6-sulfotransferase. J Biol Chem 272:32321-32328 [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. (2002). Keratan sulfate biosynthesis. IUBMB Life 54:187-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL. (2000). Keratan sulfate: structure, biosynthesis, and function. Glycobiology 10:951-958 [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Caterson B, Conrad GW. (1987). Distribution of proteoglycans antigenically related to corneal keratan sulfate proteoglycan. J Biol Chem 262:11634-11640 [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Rodrigues MM, Krachmer JH, Conrad GW. (1990). Altered antigenicity of keratan sulfate proteoglycan in selected corneal diseases. Invest Ophthalmol Vis Sci 31:419-428 [PubMed] [Google Scholar]
- Glant TT, Mikecz K, Roughley PJ, Buzas E, Poole AR. (1986). Age-related changes in protein-related epitopes of human articular-cartilage proteoglycans. Biochem J 236:71-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Milev P, Sakurai T, Karthikeyan L, Bourdon M, Margolis RK, Margolis RU. (1994). Interactions with tenascin and differential effects on cell adhesion of neurocan and phosphacan, two major chondroitin sulfate proteoglycans of nervous tissue. J Biol Chem 269:12142-12146 [PubMed] [Google Scholar]
- Habuchi O, Hirahara Y, Uchimura K, Fukuta M. (1996). Enzymatic sulfation of galactose residue of keratan sulfate by chondroitin 6-sulfotransferase. Glycobiology 6:51-57 [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Petryniak B, Kawashima H, Mitoma J, Akama TO, Fukuda MN, Lowe JB, Fukuda M. (2007). Significant decrease in alpha1,3-linked fucose in association with increase in 6-sulfated N-acetylglucosamine in peripheral lymph node addressin of FucT-VII-deficient mice exhibiting diminished lymphocyte homing. Glycobiology 17:277-293 [DOI] [PubMed] [Google Scholar]
- Hosono-Fukao T, Ohtake-Niimi S, Hoshino H, Britschgi M, Akatsu H, Hossain MM, Nishitsuji K, van Kuppevelt TH, Kimata K, Michikawa M, Wyss-Coray T, Uchimura K. (2012). Heparan sulfate subdomains that are degraded by Sulf accumulate in cerebral amyloid ss plaques of Alzheimer’s disease: evidence from mouse models and patients. Am J Pathol 180:2056-2067 [DOI] [PubMed] [Google Scholar]
- Imagama S, Sakamoto K, Tauchi R, Shinjo R, Ohgomori T, Ito Z, Zhang H, Nishida Y, Asami N, Takeshita S, Sugiura N, Watanabe H, Yamashita T, Ishiguro N, Matsuyama Y, Kadomatsu K. (2011). Keratan sulfate restricts neural plasticity after spinal cord injury. J Neurosci 31:17091-17102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Z, Sakamoto K, Imagama S, Matsuyama Y, Zhang H, Hirano K, Ando K, Yamashita T, Ishiguro N, Kadomatsu K. (2010). N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. J Neurosci 30:5937-5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander S, Schroeter M, Fischer J, Stoll G. (2000). Differential regulation of microglial keratan sulfate immunoreactivity by proinflammatory cytokines and colony-stimulating factors. Glia 30:401-410 [DOI] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. (2002). Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci 22:4611-4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe K, Tateyama D, Toyoda H, Kawasaki N, Hashii N, Nakao H, Matsumoto S, Nonaka M, Matsumura H, Hirose Y, Morita A, Katayama M, Sakuma M, Kawasaki N, Furue MK, Kawasaki T. (2013). A novel antibody for human induced pluripotent stem cells and embryonic stem cells recognizes a type of keratan sulfate lacking oversulfated structures. Glycobiology 23:322-336 [DOI] [PubMed] [Google Scholar]
- Kawashima H, Petryniak B, Hiraoka N, Mitoma J, Huckaby V, Nakayama J, Uchimura K, Kadomatsu K, Muramatsu T, Lowe JB, Fukuda M. (2005). N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol 6:1096-1104 [DOI] [PubMed] [Google Scholar]
- Krusius T, Finne J, Margolis RK, Margolis RU. (1986). Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J Biol Chem 261:8237-8242 [PubMed] [Google Scholar]
- Maeda N, Hamanaka H, Oohira A, Noda M. (1995). Purification, Characterization and Developmental Expression of a Brain-Specific Chondroitin Sulfate Proteoglycan, 6b4 Proteoglycan/Phosphacan. Neuroscience 67:23-35 [DOI] [PubMed] [Google Scholar]
- Magro G, Perissinotto D, Schiappacassi M, Goletz S, Otto A, Muller EC, Bisceglia M, Brown G, Ellis T, Grasso S, Colombatti A, Perris R. (2003). Proteomic and postproteomic characterization of keratan sulfate-glycanated isoforms of thyroglobulin and transferrin uniquely elaborated by papillary thyroid carcinomas. Am J Pathol 163:183-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L, Fritch W, Xi YG. (1997). Evolution of a strain of CJD that induces BSE-like plaques. Science 277:94-98 [DOI] [PubMed] [Google Scholar]
- Margolis RK, Rauch U, Maurel P, Margolis RU. (1996). Neurocan and phosphacan: two major nervous tissue-specific chondroitin sulfate proteoglycans. Perspect Dev Neurobiol 3:273-290 [PubMed] [Google Scholar]
- Mehmet H, Scudder P, Tang PW, Hounsell EF, Caterson B, Feizi T. (1986). The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem 157:385-391 [DOI] [PubMed] [Google Scholar]
- Meyer K, Linker A, Davidson EA, Weissmann B. (1953). The mucopolysaccharides of bovine cornea. J Biol Chem 205:611-616 [PubMed] [Google Scholar]
- Meyer-Puttlitz B, Milev P, Junker E, Zimmer I, Margolis RU, Margolis RK. (1995). Chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of nervous tissue: developmental changes of neurocan and phosphacan. J Neurochem 65:2327-2337 [DOI] [PubMed] [Google Scholar]
- Miao J, Xu F, Davis J, Otte-Holler I, Verbeek MM, Van Nostrand WE. (2005). Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am J Pathol 167:505-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Sheppard AM, Pearlman AL. (1997). Developmental expression of keratan sulfate-like immunoreactivity distinguishes thalamic nuclei and cortical domains. J Comp Neurol 380:533-552 [DOI] [PubMed] [Google Scholar]
- Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. (2012). Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci 15:414-422, S411-412. [DOI] [PubMed] [Google Scholar]
- Nakato H, Kimata K. (2002). Heparan sulfate fine structure and specificity of proteoglycan functions. Biochim Biophys Acta 1573:312-318 [DOI] [PubMed] [Google Scholar]
- Patnode ML, Cheng CW, Chou CC, Singer MS, Elin MS, Uchimura K, Crocker PR, Khoo KH, Rosen SD. (2013a). Galactose 6-o-sulfotransferases are not required for the generation of siglec-f ligands in leukocytes or lung tissue. J Biol Chem 288:26533-26545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnode ML, Yu SY, Cheng CW, Ho MY, Tegesjo L, Sakuma K, Uchimura K, Khoo KH, Kannagi R, Rosen SD. (2013b). KSGal6ST generates galactose-6-O-sulfate in high endothelial venules but does not contribute to L-selectin-dependent lymphocyte homing. Glycobiology 23:381-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas AH, West LA, Midura RJ. (2001). Keratan sulfate disaccharide composition determined by FACE analysis of keratanase II and endo-beta-galactosidase digestion products. Glycobiology 11:779-790 [DOI] [PubMed] [Google Scholar]
- Poole CA, Glant TT, Schofield JR. (1991). Chondrons from articular cartilage. (IV). Immunolocalization of proteoglycan epitopes in isolated canine tibial chondrons. J Histochem Cytochem 39:1175-1187 [DOI] [PubMed] [Google Scholar]
- Poon CJ, Plaas AH, Keene DR, McQuillan DJ, Last K, Fosang AJ. (2005). N-linked keratan sulfate in the aggrecan interglobular domain potentiates aggrecanase activity. J Biol Chem 280:23615-23621 [DOI] [PubMed] [Google Scholar]
- Rosen SD. (2004). Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 22:129-156 [DOI] [PubMed] [Google Scholar]
- Tai GH, Huckerby TN, Nieduszynski IA. (1996). Multiple non-reducing chain termini isolated from bovine corneal keratan sulfates. J Biol Chem 271:23535-23546 [DOI] [PubMed] [Google Scholar]
- Togayachi A, Kozono Y, Ishida H, Abe S, Suzuki N, Tsunoda Y, Hagiwara K, Kuno A, Ohkura T, Sato N, Sato T, Hirabayashi J, Ikehara Y, Tachibana K, Narimatsu H. (2007). Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc Natl Acad Sci USA 104:15829-15834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii T, Fukuta M, Habuchi O. (2000). Sulfation of sialyl N-acetyllactosamine oligosaccharides and fetuin oligosaccharides by keratan sulfate Gal-6-sulfotransferase. Glycobiology 10:203-211 [DOI] [PubMed] [Google Scholar]
- Uchimura K, Gauguet JM, Singer MS, Tsay D, Kannagi R, Muramatsu T, von Andrian UH, Rosen SD. (2005) A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol 6:1105-1113 [DOI] [PubMed] [Google Scholar]
- Uchimura K, Kadomatsu K, El-Fasakhany FM, Singer MS, Izawa M, Kannagi R, Takeda N, Rosen SD, Muramatsu T. (2004). N-acetylglucosamine 6-O-sulfotransferase-1 regulates expression of L-selectin ligands and lymphocyte homing. J Biol Chem 279:35001-35008 [DOI] [PubMed] [Google Scholar]
- Uchimura K, Muramatsu H, Kadomatsu K, Fan QW, Kurosawa N, Mitsuoka C, Kannagi R, Habuchi O, Muramatsu T. (1998a). Molecular cloning and characterization of an N-acetylglucosamine-6-O-sulfotransferase. J Biol Chem 273:22577-22583 [DOI] [PubMed] [Google Scholar]
- Uchimura K, Muramatsu H, Kaname T, Ogawa H, Yamakawa T, Fan QW, Mitsuoka C, Kannagi R, Habuchi O, Yokoyama I, Yamamura K, Ozaki T, Nakagawara A, Kadomatsu K, Muramatsu T. (1998b). Human N-acetylglucosamine-6-O-sulfotransferase involved in the biosynthesis of 6-sulfo sialyl Lewis X: molecular cloning, chromosomal mapping, and expression in various organs and tumor cells. J Biochem 124:670-678 [DOI] [PubMed] [Google Scholar]
- Uchimura K, Rosen SD. (2006). Sulfated L-selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol 27:559-565 [DOI] [PubMed] [Google Scholar]
- Vidal R, Barbeito AG, Miravalle L, Ghetti B. (2009). Cerebral amyloid angiopathy and parenchymal amyloid deposition in transgenic mice expressing the Danish mutant form of human BRI2. Brain Pathol 19:58-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Muramatsu T, Murase A, Yuasa S, Uchimura K, Kadomatsu K. (2006). N-Acetylglucosamine 6-O-sulfotransferase-1 is required for brain keratan sulfate biosynthesis and glial scar formation after brain injury. Glycobiology 16:702-710 [DOI] [PubMed] [Google Scholar]