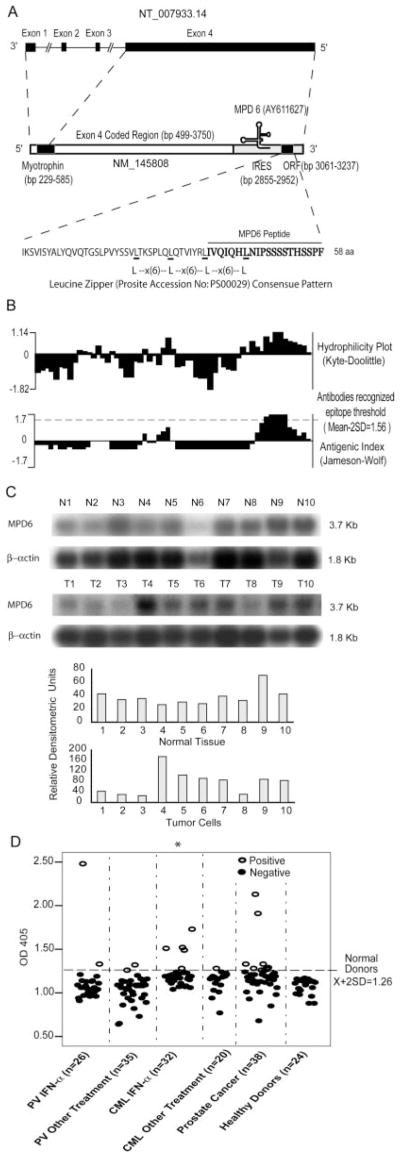
FIGURE 1.
Molecular features of MPD6. A, Schematic representation of the location of unconventional Ag MPD6 gene in the 3′-UTR of myotrophin mRNA (GenBank accession no. NM_145808) as well as the genomic structure of the myotrophin-MPD6 gene locus (GenBank accession no. NT_007933.14). The MPD6 ORF (GenBank accession no. AY611627) is located in the region of bp 3061–3237 in the 3′-UTR of myotrophin, whereas the primary ORF myotrophin is located in the region of bp 229–585. An IRES is also located in the region of the 3′-UTR of myotrophin, bp 2855–2952 upstream of MPD6 ORF. B, Feature of MPD6 protein sequence. The MPD6 ORF encodes a 58-aa protein. The start codon is an unconventional start codon AUU (isoleucine), rather than the conventional start codon AUG (methionine). Hydrophilicity plot analysis using by Kyte-Dolittle method indicated that MPD6 has a C-terminal hydrophilic region, which corresponds to the region achieving the higher Jameson-Wolf antigenic index. The Jameson-Wolf antigenic index in the MPD6 C-terminal region is higher than that of previously characterized Abs recognized epitope threshold (mean − 2SD, 1.56). MPD6 peptide used in the ELISA was synthesized according to the MPD6 sequence from aa 39 to 58. C, Higher expression of myotrophin-MPD transcripts in some tumor cells detected by Northern blots. In the upper panel, the lanes N1 to N10 indicate various normal tissues in the order of brain (N1), liver (N2), placenta (N3), small intestine (N4), colon (N5), thymus (N6), spleen (N7), prostate (N8), testis (N9), and ovary (N10), respectively. In the middle panel, the lanes T1 to T10 indicate various tumor cells in the order of acute T cell leukemia (Jurkat cells) (T1), Burkitt’s lymphoma (CA46) (T2), breast cancer (MDA-MD-453) (T3), Burkitt’s lymphoma (Namalwa) (T4), epidermal carcinoma (A-431) (T5), uterine carcinoma (MES-SA) (T6), Burkitt’s lymphoma (Raji) (T7), osterosarcoma (MG-63) (T8), histiocytic lymphoma (U-937) (T9), and cervical adenocarcinoma (Hela S3) (T10), respectively. The hybridization analyses of the normal tissue and tumor cell expression (BD Clontech) with 32P-labeled specific probes, as indicated, were performed, respectively. The transcript sizes are indicated in kilobases. The ratio of the hybridization signal density of MPD6 transcript over the hybridization signal density of β-actin in the same sample was calculated as relative densitometric unit as presented in the lower panels. D, The IgG Ab responses to the C-terminal antigenic epitope (from the aa 39 to 58) of MPD6 detected by peptide ELISA. The experiments were repeated three times, and the representative results were shown. The mean + 2 SD of the OD405 ratios of the peptide over the coating control from 24 healthy donors were calculated as the upper limit of the normal range of Ab responses to MPD6 peptide (mean + 2 SD, 1.26). The detection rates of the IgG Ab responses to MPD6 peptide in the group of CML patients treating with IFN-α are statistically higher than that of CML patients treating with other therapies (χ2 goodness-of-fit test; *, p < 0.05).