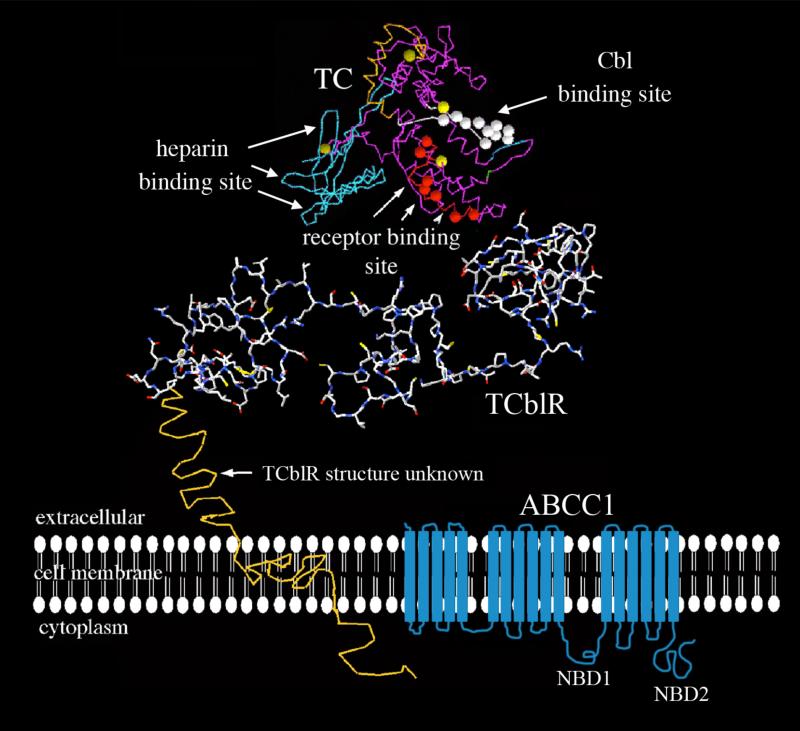
Figure 1.
The structure of proteins involved in cobalamin transport. The white circles on the TC molecule represent the Cbl binding region and the orange circles represent the receptor binding region as determined by epitope specific monoclonal antibodies that block these functions; the blue region represents the heparin-binding region likely involved in receptor binding. The TCblR molecule, with a partial theoretical 3-dimensional shape (Swiss Model) is shown oriented in the plasma membrane along with the transmembrane and cytoplasmic domains. The structure of the two LDLR-type A domains of TCblR is derived from the known structure of the LDL receptor. The two LDLR-A domains with regions involved in calcium binding are necessary for binding to holo-TC. The ABCC1 transporter involved in efflux of cobalamin is depicted in blue with its numerous transmembrane domains and nucleotide binding domains (NBD1 and NBD2).