Summary
Flow diverters (FDs) are increasingly used for complex intracranial aneurysms. As these self-expanding devices are deployed across an aneurysm neck, they can undergo deformations. The potential clinical consequences of FD deformations remain unclear.
We describe an immediate thrombotic complication attributed to a stereotypical stenotic deformation of an FD extremity that can occur when landing zones are of insufficient length. This case is supplemented with in vitro studies showing the relationship between i) the length of the landing zones and ii) discrepancies between the diameter of the device and recipient vessel, and the severity of FD stenosis.
In vitro, a shorter landing zone was associated with a progressive stenotic deformation of the terminal ends of all FDs studied. This deformation was more pronounced when the diameter of the device was oversized compared to the size of the recipient tube. In our clinical case, the presence of this deformation led to an immediate thrombotic complication, requiring deployment of a second stent to correct the observed stenosis. In addition, treatment failure ultimately led to a fatal rupture, a failure that can be explained by residual flows through a more porous transition zone, another characteristic FD deformation which occurs when they are oversized as compared to the parent vessel, but free to expand at the level of the aneurysm.
Proper selection of device diameter and length of the landing zone is important, and may decrease the incidence of deformation-related complications.
Keywords: flow diverter, aneurysm, complication, in-stent stenosis
Introduction
Flow diverters (FDs) are increasingly used in the treatment of intracranial aneurysms 1-8. Currently available FDs are flexible self-expanding devices constructed by braiding metallic filaments that can slide with respect to one another when devices are constrained to fit into a microcatheter. On deployment, the devices shorten as they are allowed to re-expand towards their unconstrained initial shape and size. One advantage of this design is that it permits the device to conform to the local arterial anatomy. However, in certain circumstances, this device expansion can lead to stereotypical deformations that have recently been characterized 3.
When the device is oversized, i.e. its diameter is larger than the parent artery, it is free to expand in its mid-portion when it spans a large arterial defect, such as an aneurysm ostium. This expansion causes a fusiform deformation to develop, which is accompanied by a quantifiably more porous transition zone at the junction between the expanded segment at the aneurysm ostium and the constrained segment at the proximal and distal landing zones of the device 3,4. This fusiform deformation and the porosity alterations that accompany it can be responsible for treatment failure, as blood leaks into the aneurysm through the increased porosity transition zone.
Furthermore, when the landing zone is short and the flow diverter oversized compared to the parent artery, the terminal extremity of the device can become conically deformed and a stenosis ensues 3. This stenosis may cause immediate thromboembolic complications, as reported here. This stenotic problem can be reproduced in vitro and potentially prevented by careful sizing and provision of sufficiently long landing zones upon deployment of the device.
Here, we describe a clinical case of a complex aneurysm treated with a FD, where device deformation was associated with an immediate thrombo-embolic complication, a delayed recurrence and fatal rupture of the aneurysm. The clinical case is supplemented by explanatory in vitro studies.
Case Report
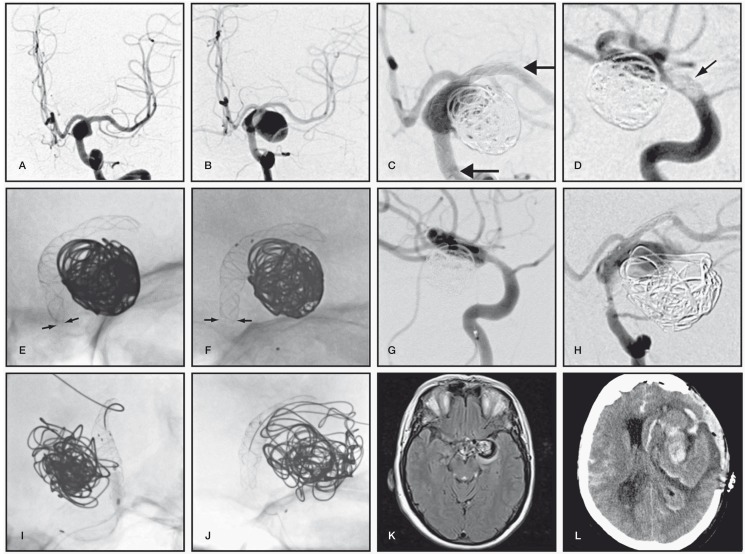
A 70 year-old asymptomatic woman, diagnosed several years earlier with a 9 mm fusiform left supraclinoid carotid artery aneurysm, was subsequently found on surveillance imaging to have an enlarging lesion with a new sacculation (Figure 1A,B). The lesion had progressed to 18 × 13 mm with a 15 mm neck; the diameter of the ICA at the level of the ophthalmic artery was 3.9 mm, and the diameter of M1 was 3.0 mm. She remained asymptomatic, but the change in morphology was felt to be sufficiently concerning to warrant aneurysm treatment with coiling of the sac and flow diversion. The patient was prepared with standard dual antiplatelet therapy (Clopidogrel 75 mg and ASA 80 mg) for five days. Testing for clopidogrel response was not performed. After femoral puncture, a 5000 IU heparin IV bolus was administered and the ACT kept above 300 seconds throughout the procedure. Using a balloon-assisted technique (4 × 20 mm Scepter balloon; Microvention, Tustin, CA, USA) and an Excelsior 1018 microcatheter (Stryker, Kalamazoo, MI, USA), a total of 167 cm of platinum coils (One Microplex 18 Cosmos and four Microplex 10 VFC; Microvention, Tustin, CA, USA) was inserted to densely pack the aneurysm fundus while maintaining loose packing in the region of the neck. To deploy the FD, the left MCA was catheterized with a Vasco 21+ (Balt, Montmorency, France), and a 4 × 30 mm Silk (Balt, Montmorency, France) was deployed spanning the proximal M1 segment back to the distal ophthalmic segment of the carotid artery (Figure 1C). The FD was seen to expand at the level of the aneurysm. Antegrade blood flow through the device was maintained, and the procedure terminated.
Figure 1.
A) Initial and B) 4-year follow-up angiography demonstrating interval growth of left supraclinoid carotid aneurysm. C) Coil embolization followed by deployment of a Silk flow diverter. Arrows show FD extremities. D) Lateral carotid angiography 2 hours later showing thrombus on the proximal end of the Silk, which had become stenosed (E). F) Solitaire stenting to correct proximal Silk stenosis. Arrows in (E) show the diameter of stenosis of the proximal FD corrected by a Solitaire stent (arrows in F). G) Immediate post-treatment angiography. H) Follow-up angiography at 9 months, showing major saccular recurrence with significant proximal in-stent stenosis. I) Balloon angioplasty followed by J) deployment of a second Silk flow diverter. K) Axial MRI sequences 2 months later demonstrating enlarging recurrence with peri-aneurysmal edema, which was followed by rupture and fatal re-rupture (J) two months later.
Approximately two hours following the intervention, the patient suddenly became hemiparetic and aphasic. An urgent CT scan of the head was negative for hemorrhage thus the patient was taken back to the neurointerventional suite. Angiography demonstrated a filling defect at the proximal end of the FD, consistent with thrombus (Figure 1D). A 0.25 mg/kg (18 mg) intra-arterial bolus of Abciximab (Reopro, Lilly, IN, USA) was followed by a 0.125 mcg/kg/minute intravenous infusion for a total dose of 27 mg. Close study of the images showed that the proximal FD was not properly apposed to the arterial wall, and the proximal landing zone of the FD was shortened to only 6-7 mm with an associated stenotic deformity (Figure 1E). Several attempts to catheterize the FD with a Rebar 21 microcatheter (ev3 Inc, Plymouth, MN, USA) and a Terumo 16 guidewire failed. On most occasions, the guidewire reached the aneurysm, passing between the proximal stent extremity and the wall of the carotid artery, because the end of the Silk was not fully opened. The lumen of the FD was finally catheterized, and a 4 × 20 mm Solitaire FR stent (Covidien, Irvine CA, USA) was deployed from the mid-section of the FD to the proximal carotid artery and detached in situ. The Solitaire stent improved the diameter of the proximal Silk extremity from 3.2 to 3.9 mm (Figure 1F). The final angiogram showed persistence of a small non-occlusive mural clot without distal emboli (Figure 1G). Post-procedure, the patient had a transient mild right hemiparesis and aphasia, with full recovery within two days. Delayed post-procedural imaging did not show any cerebral infarction. She was discharged four days after her procedure, with dual antiplatelet therapy, and at three months, her mRS score remained at 0.
Nine months later, the patient returned with left hemispheric TIAs. Angiography revealed narrowing of the distal M1 segment of the Silk device, a 60% in-stent stenosis of the proximal segment of the construct, where the Solitaire and Silk overlapped, and a significant recurrence of the aneurysm (Figure 1H). The leak was mainly located at the distal transition zone between the expanded and constrained segments of the construct. The proximal stenosis was dilated using a 2.5 × 10 mm Gateway balloon (Figure 1I); the M1 segment was re-catheterized with a Vasco 21+ and an overlapping 3.5 × 20 mm Silk deployed in stent-in-stent fashion (Figure 1J). Follow-up MRI-MRA 12 months after the initial procedure showed an increase in aneurysm diameter from 22 to 30 mm, accompanied by progressive surrounding parenchymal edema (Figure 1K). Two weeks later, while in the pre-procedure area awaiting an angiogram, the patient complained of a severe headache, followed by right hemiplegia, aphasia, and a decrease in her level of consciousness. After confirming an intraparenchymal hemorrhage on CT scan of the head, the patient was intubated. She subsequently developed a fixed and dilated left pupil. Urgent surgical hematoma evacuation, combined with a large decompressive craniectomy was performed, but the patient nonetheless died three days later (Figure 1L).
In Vitro Studies
Methods
A 4 × 30 mm Silk flow diverter, identical to the one initially used in the case reported, was first studied in vitro. To study the terminal portion of the flow diverter, a segment of the device (the landing zone) was deployed into silicone tubes of 2.0, 2.5 and 3.0 mm to simulate undersized arteries of various diameters, leaving the rest of the FD free to expand unconstrained outside the tube. The stent was progressively retrieved, thus shortening the landing zone, and allowed to adapt to the geometry of the setup. For each new landing zone length, a scaled photograph of the terminal stent was taken using a stereo-microscope, and the diameter of the terminal, in-tube portion of the device was measured. The degree of terminal stent stenosis was determined by dividing the terminal stent diameter by the tube (vessel) diameter, and was correlated to the length of the landing zone. To mimic the clinical case, similar experiments were performed in the presence of a curve. Finally, experiments were repeated using a 4 × 20 mm Pipeline Flow Diverter, a 3.75 × 33 mm FRED Flow Diverter 36 and a 3.75 × 32 mm FRED Flow Diverter 64 (all gifts from Microvention).
Results
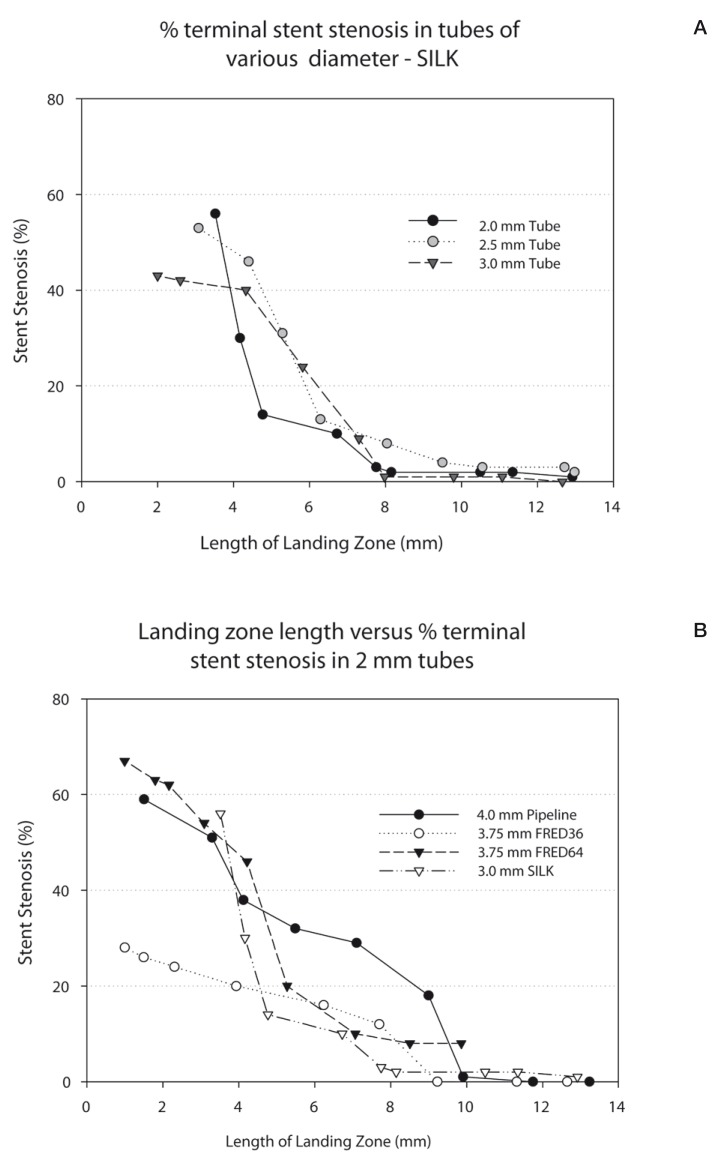
A decrease in the length of the landing zone led to a decrease in the diameter of the terminal portion of the FD, and therefore an increase in the percentage of terminal stent stenosis (Figure 2A,B). This phenomenon was more pronounced in tubes of smaller calibre (Figure 3A) and occurred with all of the braided FD devices studied (Figure 3B).
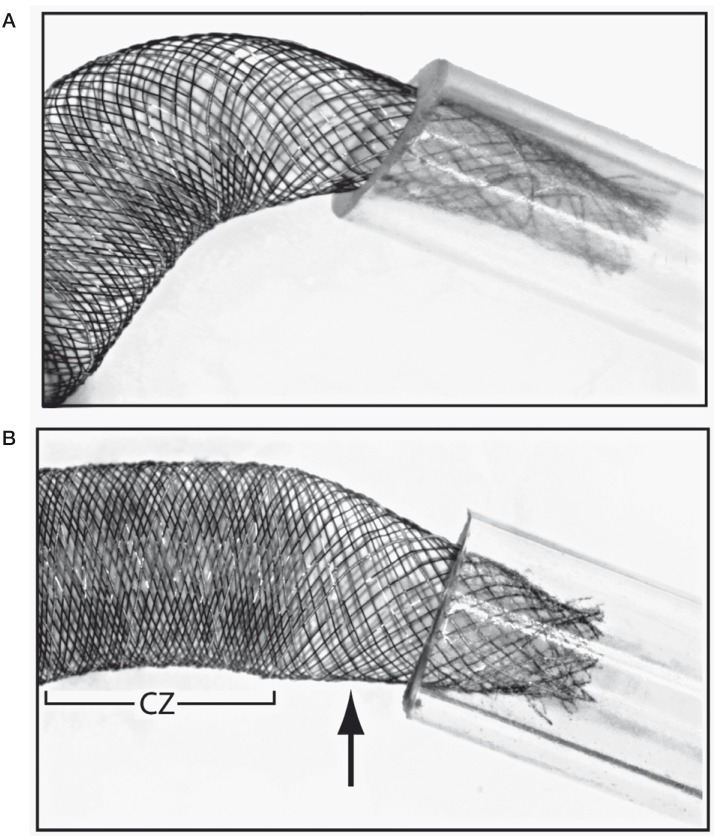
Figure 2.
A) The 4 × 30 mm Silk is constrained in a 2.5 mm silicone tube; despite the presence of a 6 mm landing zone, there is a 20% stenosis of the extremity of the device (as compared to tube diameter). B) The 4 × 30 mm Silk is constrained in a 3.5 mm silicone tube with a 3 mm length landing zone, demonstrating a 40% eccentric stenosis. In this image, note the increased porosity of the transition zone (arrow) compared to the adjacent compaction zone (CZ).
Figure 3.
A) Graph showing the relationship between the length of the landing zone and stent stenosis, when the Silk stent is constrained within tubes of various diameters. B) Graph showing the relationship between the length of the landing zone and stent stenosis, when various flow diverters are constrained within a 2 mm tube.
In a 3 mm tube, stenosis of the Silk extremity (the device used in the patient reported) did not occur when the landing zone was 8 mm (1%); it was minimal (10%) at 7 mm, slightly more pronounced at 6 mm (24%), and moderate at 4 mm (40%) (Figure 3A).
Discussion
The main finding of this report is that upon deployment of oversized flow diverters, landing zones of insufficient length may lead to device deformation with minimal or moderate terminal stenosis. Even though the stenosis may not be flow-limiting, the poor apposition of the device to the wall of the parent vessel may cause thrombo-embolic complications. Furthermore, deformation of the FD can be associated with a more porous transition zone that can explain failures and recurrences. The reported case illustrates both an immediate thrombo-embolic complication as a result of proximal device stenosis, and delayed failure of aneurysm occlusion at the level of the distal transition zone.
When treating complex, wide-necked aneurysms, the diameters of the proximal and distal parent vessels can be substantially different. The device is often chosen to match the diameter of the usually larger more proximal vessel, which may contribute to device deformation. Stents of tapering diameter may potentially correct this discrepancy. Devices designed with flared extremities may also minimize terminal stenoses. In general, short landing zones are often seen as desirable to decrease the number of jailed branches or perforating arteries. Here we see that these choices can lead to significant terminal device stenosis and thrombus formation. In vitro, a landing zone more than twice the diameter of the parent vessel (6-8 mm on each side), a longer length than is typically recommended, was still associated with 10-20% stenoses of all devices examined. However, it is important to remember that longer landing zones may increase the risks of perforator and branch occlusion 9-10.
Although thrombo-embolic complications during or immediately after interventions can be successfully treated with Abciximab 11, this rescue therapy is not without risk. Other treatments in the setting of acute thrombo-embolic complications include stent-trievers, such as the Solitaire stent, which are increasingly used in the management of acute stroke 12. In this case, the use of the stent was to mechanically correct the proximal stenosis of the flow diverter.
Placement of coils within the fundus of an aneurysm prior to flow diversion has been proposed to decrease the haemorrhagic risks that may be associated with flow diversion 13. Coiling or even stenting may also offer some support to minimize the phenomenon of FD expansion, although in this case, coiling did not prevent deformation and stenosis.
It is tempting to correlate the failure of the aneurysm to become occluded with our in vitro studies showing the constant presence of a more porous transition zone on each side of the zone which expands in a fusiform fashion (Figure 3B) 3. Admittedly, the second FD deployed at the time of retreatment did not fully cover the distal transition zone of the first FD (the device did not reach M1) because we were concerned regarding the amount of metal being implanted at the level of an already stenosed MCA. This is a possible explanation of the observed failure of retreatment, with persistent aneurysm expansion and eventual rupture. An alternative potential explanation is related to the choice of diameter of the second Silk device, which was 0.5 mm smaller than the first Silk. Although the choice of a smaller device can theoretically lead to endoleaks, we do not think this mechanism was responsible in this case, because the 3.5 mm Silk expands to 4.0 mm when unconstrained, and the first 4mm Silk was already constrained to less than 4mm. Either way, the large, fatal recurrence of this aneurysm despite the presence of coils, a high porosity stent, intra-stent stenosis and two FDs may be another occasion to question our understanding of how flows, healing and recurrences are related after endovascular treatment 14.
Kulscar et al. 10 described four features common to aneurysms which ruptured two days to five months following flow diversion: a) large or giant size; b) symptomatic lesions; c) saccular morphology with AR > 1.6 (mean AR of 3.1 ± 0.9); and d) inertia-driven aneurysmal inflow. They also hypothesized that intra-aneurysmal thrombosis was a possible cause of delayed aneurysm rupture. The hemorrhage occurred more than one year after flow diversion in the present report, after treatment failure and aneurysm growth had been documented. Recurrences after coiling, stent-assisted coiling, or after flow diversion and coiling are always possible, and one may wonder if this is not one of those aneurysms that recurs no matter what treatment is used. Thus we believe that, in the presence of a large recurrence, no other or new mechanistic hypothesis is necessary to explain the delayed rupture in this particular case.
Conclusions
The self-expanding nature of flow-diverting stents leads to a predictable deformation of the device. The observed fusiform dilatations of the device can have two untoward effects: terminal device stenosis with thrombus formation, and formation of a higher-porosity transition zone, which can lead to aneurysm recurrences. Careful device selection and provision of sufficient landing zones are paramount to minimize complications.
Acknowledgments and Funding
This work was supported by a grant from Fonds de la Recherche du Québec – Santé to JR. The Authors declare no conflict of interest.
References
- 1.Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62(5):1115–1120;. doi: 10.1227/01.neu.0000325873.44881.6e. discussion 1120-1111. [DOI] [PubMed] [Google Scholar]
- 2.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632–642. doi: 10.1227/01.NEU.0000339109.98070.65. discussion 642-633; quiz N636. [DOI] [PubMed] [Google Scholar]
- 3.Makoyeva A, Bing F, Darsaut TE, et al. The varying porosity of braided self-expanding stents and flow diverters: An experimental study. Am J Neuroradiol. 2013;34(3):596–602. doi: 10.3174/ajnr.A3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bing F, Darsaut TE, Salazkin I, et al. Stents and flow diverters in the treatment of aneurysms: device deformation in vivo may alter porosity and impact efficacy. Neuroradiology. 2013;55(1):85–92. doi: 10.1007/s00234-012-1082-0. [DOI] [PubMed] [Google Scholar]
- 5.McAuliffe W, Wycoco V, Rice H, et al. Immediate and midterm results following treatment of unruptured intracranial aneurysms with the pipeline embolization device. Am J Neuroradiol. 2012;33(1):164–170. doi: 10.3174/ajnr.A2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson PK, Lylyk P, Szikora I, et al. The Pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol. 2011;32(1):34–40. doi: 10.3174/ajnr.A2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pistocchi S, Blanc R, Bartolini B, et al. Flow diverters at and beyond the level of the circle of Willis for the treatment of intracranial aneurysms. Stroke. 2012;43(4):1032–1038. doi: 10.1161/STROKEAHA.111.636019. [DOI] [PubMed] [Google Scholar]
- 8.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. Am J Neuroradiol. 2010;31(6):1139–1147. doi: 10.3174/ajnr.A2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puffer RC, Kallmes DF, Cloft HJ, et al. Patency of the ophthalmic artery after flow diversion treatment of paraclinoid aneurysms. J Neurosurg. 2012;116(4):892–896. doi: 10.3171/2011.11.JNS111612. [DOI] [PubMed] [Google Scholar]
- 10.Kulcsár Z, Houdart E, Bonafé A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol. 2011;32(1):20–25. doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tähtinen OI, Manninen HI, Vanninen RL, et al. The Silk flow-diverting stent in the endovascular treatment of complex intracranial aneurysms: technical aspects and midterm results in 24 consecutive patients. Neurosurg. 2012;70(3):617–623. doi: 10.1227/NEU.0b013e31823387d4. Discussion 623-614. [DOI] [PubMed] [Google Scholar]
- 12.Costalat V, Machi P, Lobotesis K, et al. Rescue, combined, and stand-alone thrombectomy in the management of large vessel occlusion stroke using the solitaire device: a prospective 50-patient single-center study: timing, safety, and efficacy. Stroke. 2011;42(7):1929–1935. doi: 10.1161/STROKEAHA.110.608976. [DOI] [PubMed] [Google Scholar]
- 13.Urgent Field Safety Notice: Intracranial stent ‘SILK.’ Clarifications of the indications. Letter to the intention of Hospital Chief Executives, Medical Directors and Directors of Radiology. Balt Extrusion; 2010. Accessed on September 1st, 2010. Available from: http://www.mhra.gov.uk/home/groups/dts-bi/documents/fieldsafetynotice/con076110.pdf. [Google Scholar]
- 14.Raymond J, Darsaut T, Salazkin I, et al. Mechanisms of occlusion and recanalization in canine carotid bifurcation aneurysms embolized with platinum coils: an alternative concept. Am J Neuroradiol. 2008;29(4):745–752. doi: 10.3174/ajnr.A0902. [DOI] [PMC free article] [PubMed] [Google Scholar]