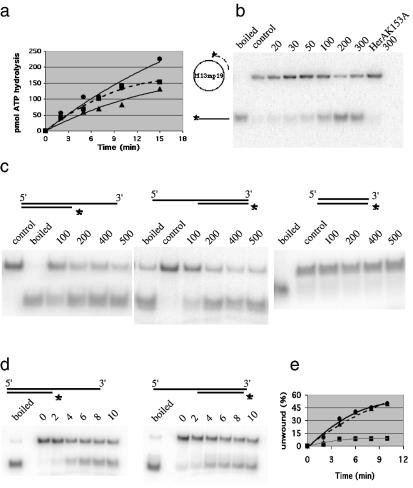
Figure 5.
ATPase and helicase activities associated with HerA protein. (a) Time course of HerA ATPase activity: ATP hydrolysis was followed at 70°C for the indicated times in the absence of DNA (triangles) or in the presence of single-stranded DNA (squares) or double-stranded DNA (circles). (b) Helicase activity recovered with a primed circular single-stranded DNA substrate and 20–300 nM wild-type HerA protein or 300 nM HerAK153A protein in 30 min at 70°C. (c) Helicase activity recovered after a 30 min incubation at 70°C using 5′ overhang, 3′ overhang or blunt linear DNA substrates in the presence of 100–500 nM HerA protein. (d and e) Time course of HerA helicase activity. HerA (400 nM) was incubated at 70°C with 5′ overhang (triangles) or 3′ overhang (circles) DNA substrates for the time points shown. Squares correspond to the quantification of the control experiment [data not shown in (d)] performed without enzyme. In (b) and (c), control lanes correspond to the DNA substrate incubated for 30 min at 70°C without enzyme and in (b), (c) and (d), boiled lanes correspond to the heat-denatured substrate.