Figure 2.
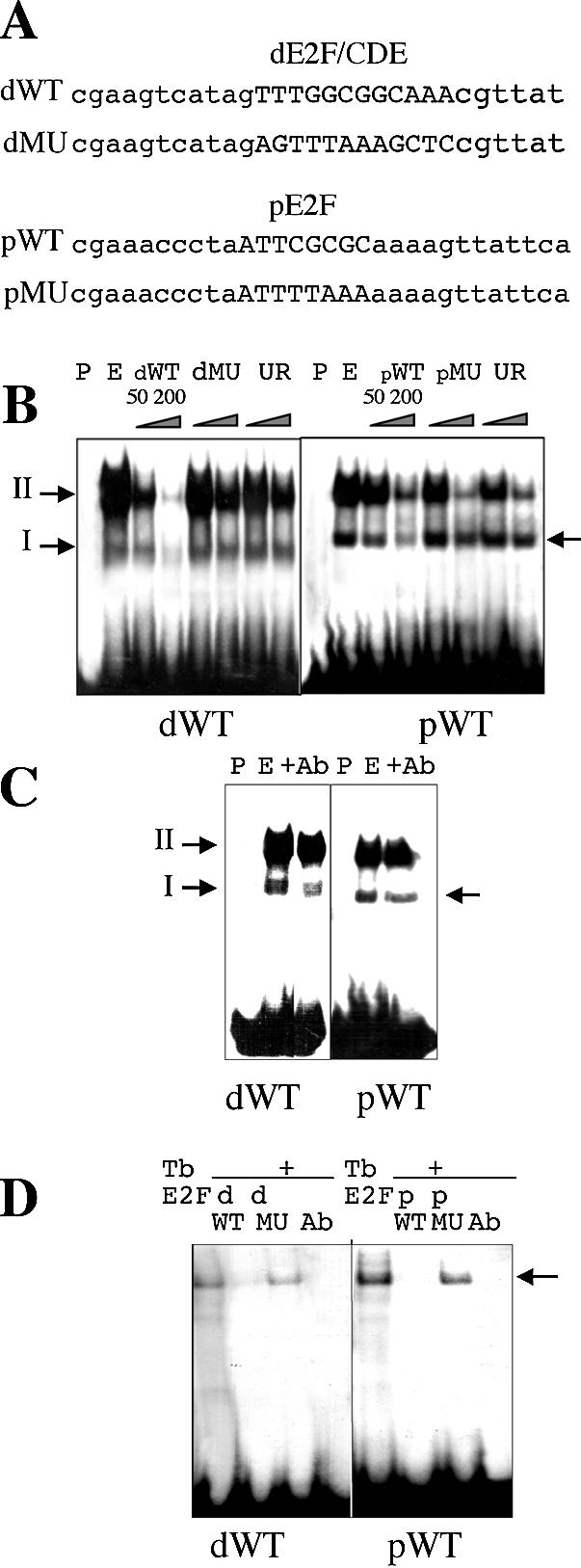
Binding properties of the E2F elements of the RNR1a promoter. (A) dWT and pWT oligonucleotides carrying either dE2F/CDE or pE2F motifs are indicated as well as their mutated versions in their E2F sites, dMU and pMU, respectively. (B) The WT oligonucleotides were used as probes (P) in gel shift experiments performed with nuclear extracts (E) prepared from mid-log phase BY-2 cells. The dWT probe revealed two specific complexes, I and II, whose binding was competed by a 50–200-fold molar excess of unlabelled oligonucleotides (dWT) but not by the E2F-mutated (dMU) and unrelated (UR) oligonucleotides. In contrast, a single specific complex was revealed with the pWT probe (see arrow): binding is competed by an excess of the pWT oligonucleotides but not by the pMU oligonucleotides. (C) E2F factor is part of nuclear complexes bound to E2F elements of the RNR1a promoter. Binding of specific complexes is competed by 2 µl of antibody directed against the DBD of human E2F5 (Ab). (D) Specific interaction between E2F sites of the RNR1a promoter and a purified tobacco E2F factor (TbE2F). A complex is detected (see arrow) with both WT probes and the complex is competed by a 200-fold molar excess of the wild-type unlabelled probe (pWT or dWT) and 2 µl of antibody raised against the DBD of human E2F5 (Ab) but not by a 200-fold excess of E2F-mutated oligonucleotides (dMU or pMU).
