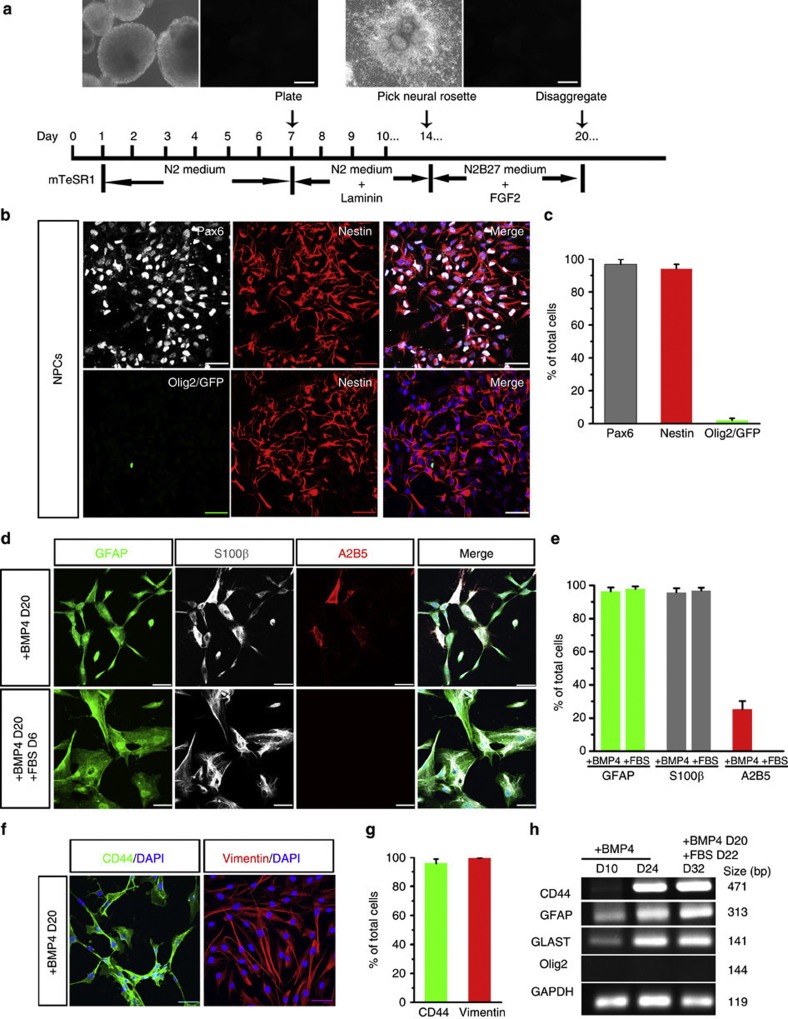
Figure 2. Differentiation of astroglial cells from Olig2-negative NPCs.
(a) A schematic procedure for differentiating Olig2-GFP hESCs to NPCs. Insets: left, bright field and GFP channel demonstrating that GFP fluorescence is undetectable at day 7 in the absence of RA and Pur. Right, bright field and GFP channel showing that at day 14, the EBs become flat after plating and neural rosettes were observed, but GFP fluorescence is undetectable. These neural rosettes are ready to be manually picked up and further cultured in suspension. Scale bars represent 300 μm. (b and c) Representative and quantification of Pax6, nestin, and Olig2/GFP-expressing cells among the cells disaggregated from neurospheres at D20 (n=3). Noticeably, very few cells are Olig2+/GFP+. (d and e) Representative and quantification of GFAP-, S100β-, and A2B5-expressing cells when the NPCs are induced to astroglia by BMP4 or FBS (n=3). (f and g) Representatives and quantification of CD44- and vimentin-expressing cells when cultured with medium containing BMP4 (n=3). (h) RT-PCR data showing expressions of CD44 (471 bp), GFAP (313 bp), GLAST (141 bp) and Olig2 (144 bp) in NPC-Astros cultured with BMP4 or FBS. Data are presented as mean±s.e.m. Blue, DAPI-stained nuclei. Scale bars in all panels represent 50 μm.