Fig. 3.
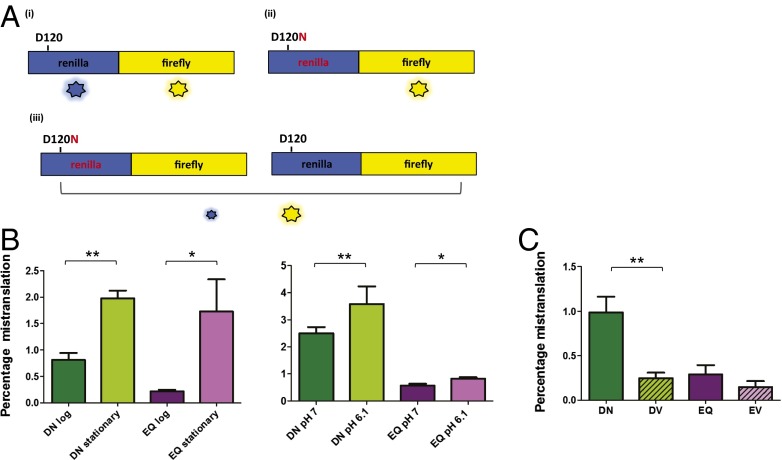
Mycobacteria have high rates of misincorporation of glutamate and aspartate. (A) Schematic of the dual-luciferase mistranslation reporter based on ref. 23. The reporter is a fusion protein of luciferase enzymes from Renilla reniformis (blue, Renilla) and Photinus pyralis (yellow, Firefly). The WT enzyme exhibits luciferase activity from both enzymes (i). If an active site residue of the Renilla enzyme is mutated to render it inactive (e.g., D120N), the firefly enzyme retains activity, but the Renilla enzyme now has no activity (ii). However, if the mutated enzyme from ii is expressed under conditions of low translational fidelity, a proportion of the protein will be translated with a reconstituted active site, which results in some measurable activity (iii). The firefly activity controls for total protein expression and thus allows accurate measurement of mistranslation rates. (B) Amino acid misincorporation rates under different physiological conditions. Misincorporation of aspartate for asparagine in stationary phase (DN stationary) is higher than in log phase (DN log) and similarly for glutamate misincorporation for glutamine (EQ). Amino acid misincorporation is higher at pH 6.1 compared with pH 7.0. (C) Mistranslation rates of aspartate or glutamate for valine are lower than aspartate for asparagine and glutamate for glutamine (*P < 0.05, **P < 0.01, Student t test).