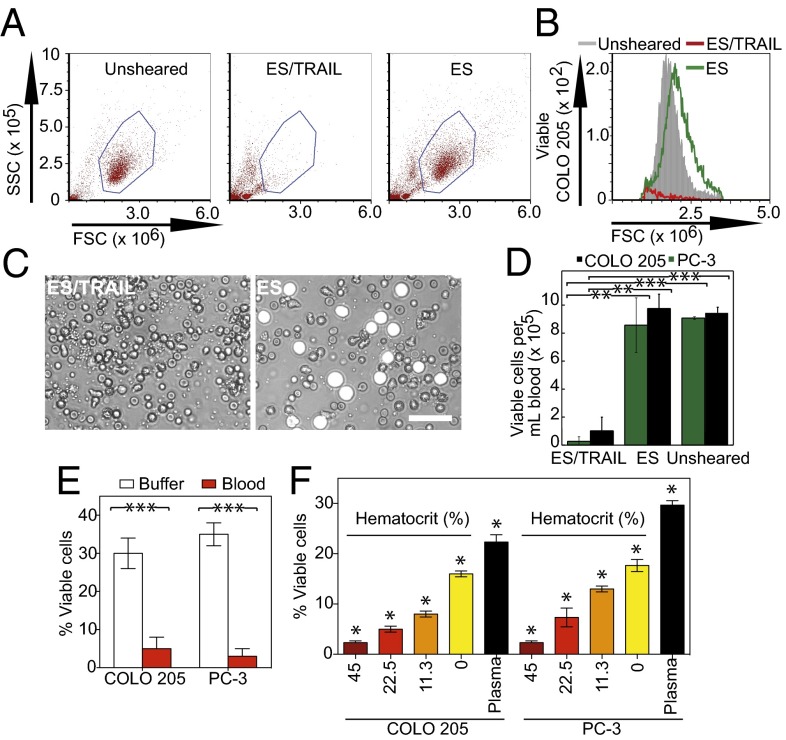
Fig. 3.
ES/TRAIL liposome therapeutic effects are enhanced in human blood under flow in vitro. (A) Flow cytometry of COLO 205 cancer cells after treatment with ES/TRAIL or ES liposomes in blood under shear flow in a cone-and-plate viscometer at 188 s−1 for 2 h. Unsheared, viable untreated cancer cell control. (B) Representative flow cytometry histogram showing the number of viable cancer cells collected. (C) Representative micrographs of COLO 205 cells (white) in blood when treated with ES/TRAIL (Left) and ES only (Right) liposomes in blood under shear flow. (Scale bar, 50 μm.) (D) Number of viable COLO 205 and PC-3 cells per volume of blood after treatment with ES/TRAIL or ES liposomes in blood under shear flow. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. **P < 0.001, ***P < 0.0001 (unpaired t test). (E) Comparison of fraction of COLO 205 and PC-3 cells that remained viable after treatment with ES/TRAIL liposomes in buffer versus blood. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. ***P < 0.0001 (unpaired t test). (F) Fraction of COLO 205 and PC-3 cells that remained viable after treatment with ES/TRAIL liposomes in blood with varying percentages of normal hematocrit. Hematocrit was varied whereas other blood components remained constant, based on a normal hematocrit of 45%. Plasma indicates removal of all blood cells. n = 3 for all samples. Bars represent the mean ± SD in each treatment group. *P < 0.05 (one-way ANOVA with Tukey posttest).