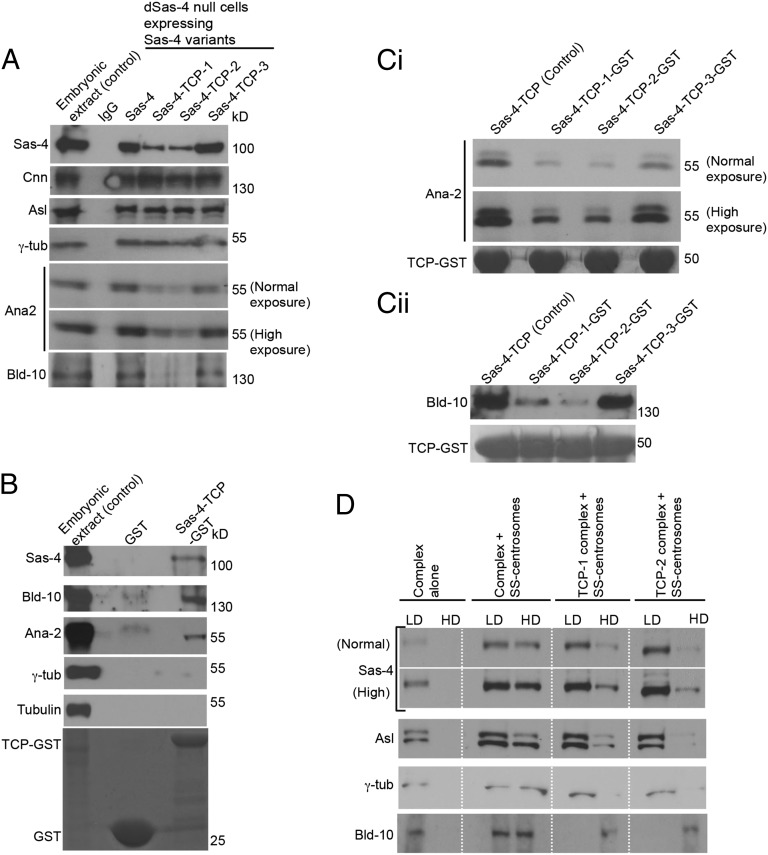
Fig. 2.
The β9–10 surface of TCP domain mediates an interaction with Bld-10 and Ana2 and is required to tether Sas-4–PCM scaffolds in a centrosome. (A) Sas-4–TCP variants can assemble cytoplasmic complexes of centrosomal proteins. Immunopurified Sas-4–TCP variants containing complexes from C131 cells reveal the presence of S-CAP components. Variants TCP-1 (E792V) and TCP-2 (V800A/I802A) specifically fail to bind Ana2 and Bld-10. Embryonic extracts were used as a positive control and mouse IgG-coated beads were used as a negative control. (B) GST pull-down assay. TCP domain alone pulls down Ana2 and Bld-10 from Drosophila embryonic extracts. Embryonic extracts were used as a positive control. (C) In a GST pull-down assay, TCP-1 (E792V) and TCP-2 (V800A/I802A) variants bind a reduced amount of Ana2 (Ci) and Bld-10 (Cii), compared with wild type or TCP-3 (L841K) variant. Both normal and high exposures are shown. (D) Cell-free binding assay of purified Sas-4–TCP variants containing complexes to stripped centrosomes by discontinuous sucrose-gradient centrifugation. Unbound complexes are found in the low-density (LD) fractions and bound complexes in the high-density (HD) fractions that contain stripped centrosomes. In the absence of stripped centrosomes (complexes alone), the components of Sas-4 complexes (Sas-4, Asl, γ-tubulin, and Bld-10) remain only in the low-density fractions. Control Sas-4 complexes but not TCP-1 (E792V)- or TCP-2 (V800A/I802A)-containing complexes sink efficiently with Bld-10–positive stripped centrosomes that are detected in the high-density fractions by Western blots. Control and TCP-1 (E792V)- or TCP-2 (V800A/I802A)-containing complexes were prepared as stated in A. Bld-10–positive stripped centrosomes were prepared as mentioned in SI Appendix, Fig. S8. TCP3 variant was not used in this experiment because this variant behaved like the wild-type control.