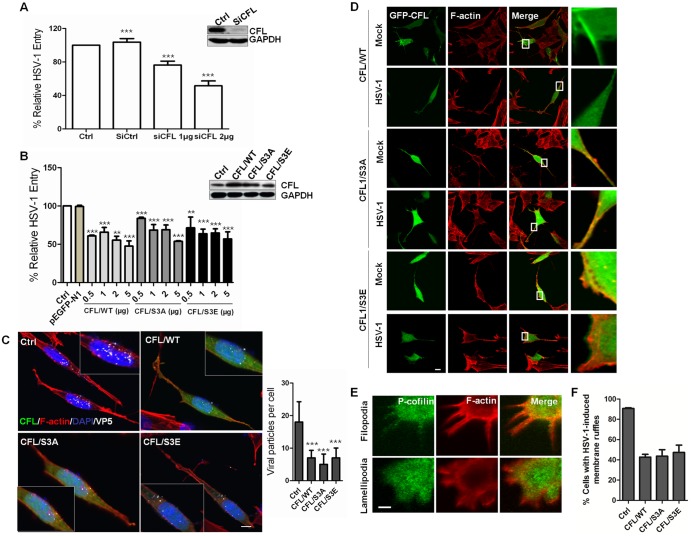
FIG 2 .
Role for cofilin in HSV-1-mediated membrane ruffling and entry. (A and B) HSV-1 entry is impaired by cofilin knockdown (A) or the transient overexpression of wild-type cofilin or mutants (S3A and S3E) (B). Western blot results show the knockdown or overexpression efficacy. Cells were transfected with siRNA or plasmids. At 24 h postinfection, the cells were infected with HSV-1 (MOI =20) for 1 h, and HSV-1 entry was evaluated by real-time PCR. The results are representative of three separate experiments. Ctrl, control. (C) Confocal microscopy assays showing inhibited virus entry. The cells were transfected with green fluorescent protein (GFP)-tagged plasmids (2 µg), infected with HSV-1 (MOI 20) for 1 h, fixed, and then stained with anti-ICP5 (white). Bound virions that had not entered the cells were removed by washing with cold PBS (pH 3.0). The average number of ICP5-positive capsids per nucleus was determined to evaluate viral entry. At least 50 cells with ICP5 docked at nuclei from 5 representative fields were counted in each independent experiment. (D) Active cofilin motivation during HSV-1 intracellular trafficking. The cells were infected for 60 min, stained for F-actin (red) or cofilin (green), and examined by LSM. Viral ICP5 was stained (not shown) to distinguish the infected cells from the mock-infected cells. (E) Colocalization between p-cofilin and F-actin at filopodia and lamellipodia. The cells were infected with HSV-1 and stained with either anti-p-cofilin MAb (green) or TRITC-phalloidin (red). Areas of colocalization appear yellow. (F) Overexpression of cofilin reduces the HSV-1-mediated production of cell ruffles. Cells with filopodia or lamellipodia were designated positive cells, and at least 50 cells from five representative fields were counted in each experiment.