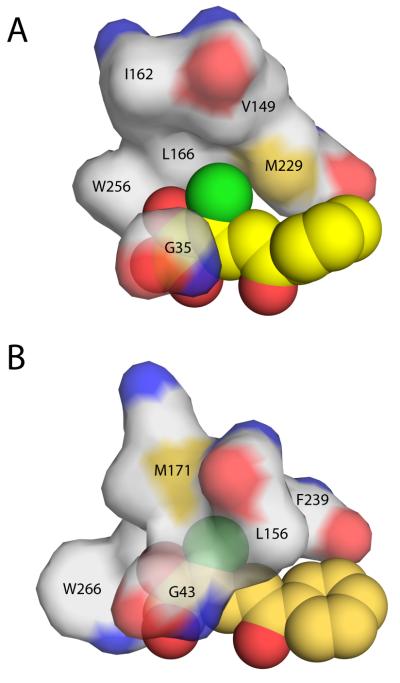
Figure 7.
Protein surface representation of the hydrophobic pocket that stabilizes the planar 3-Cl HOPDA binding mode in (A) DxnB2 S105A and (B) BphDLB400 S112A (PDB ID: 2RHT). Leu156 of the BphDLB400 lid domain is the positional equivalent of Val149 in DxnB2, which is further removed from the MCP bound in the dimeric homolog. Other DxnB2 lid domain residues illustrated include Ile162 (BphDLB400 Met171) and Leu166. The remaining residues that help to form the hydrophobic pocket are part of the α/β-hydrolase core domain.