Abstract
Here we investigate the role of hypoxia inducible factor (HIF)-2α in coordinating the development of retinal astrocytic and vascular networks. Three Cre mouse lines were used to disrupt floxed Hif-2α, including Rosa26CreERT2, Tie2Cre, and GFAPCre. Global Hif-2α disruption by Rosa26CreERT2 led to reduced astrocytic and vascular development in neonatal retinas, whereas endothelial disruption by Tie2Cre had no apparent effects. Hif-2α deletion in astrocyte progenitors by GFAPCre significantly interfered with the development of astrocytic networks, which failed to reach the retinal periphery and were incapable of supporting vascular development. Perplexingly, the abundance of strongly GFAP+ mature astrocytes transiently increased at P0 before they began to lag behind the normal controls by P3. Pax2+ and PDGFRα+ astrocytic progenitors and immature astrocytes were dramatically diminished at all stages examined. Despite decreased number of astrocyte progenitors, their proliferation index or apoptosis was not altered. The above data can be reconciled by proposing that HIF-2α is required for maintaining the supply of astrocyte progenitors by slowing down their differentiation into non-proliferative mature astrocytes. HIF-2α deficiency in astrocyte progenitors may accelerate their differentiation into astrocytes, a change which greatly interferes with the replenishment of astrocyte progenitors due to insufficient time for proliferation. Rapidly declining progenitor supply may lead to premature cessation of astrocyte development. Given that HIF-2α protein undergoes oxygen dependent degradation, an interesting possibility is that retinal blood vessels may regulate astrocyte differentiation through their oxygen delivery function. While our findings support the consensus that retinal astrocytic template guides vascular development, they also raise the possibility that astrocytic and vascular networks may mutually regulate each other's development, mediated at least in part by HIF-2α.
Introduction
Retinal vascular development is closely associated with the development of an astrocytic template. Earlier studies found that retinal astrocytes were present in animal species with vascularized retinas but absent in animals with avascular retinas [1]. More recent studies employed gene targeting approach in mice to address the relationship between astrocytic and vascular development. Tlx null mutation led to poor astrocytic and vascular development in the retina [2]. Besides reduced astrocyte numbers, Tlx null mice also displayed poor assembly of extracellular fibronectin matrices [3], and astrocyte specific Tlx disruption further demonstrated that the expression of both fibronectin and heparan-sulfate was compromised [4]. These extracellular components were thought to mediate retinal vascularization by regulating VEGF-A binding and distribution [4].
In rodents, the development of retinal astrocyte network begins at birth with immigration of Pax2-positive cells from the optic nerve, spreading in a centrifugal direction in the retinal inner surface [5], [6]. Pax2+ cell population gives rise to both optic nerve astrocytes and retinal astrocytes, with the progenitors to the latter also expressing PDGFRα in addition to Pax2 [7]. PDGFRα expression is critical to the proliferation of immature retinal astrocytes in response to stimulation by PDGFA from retinal ganglion cells [8]. As astrocyte progenitors migrate towards the retinal periphery, vascular structures emerge from the optic nerve, forming a vascular network which expands towards retinal periphery behind the PDGFRα+ astrocytic network. In vascularized areas, astrocyte maturation occurs, presumably mediated by endothelial cell derived leukemia inhibitory factor (LIF) [9]–[11]. Mature astrocytes exhibit high level expression of glial fibrillary acidic protein (GFAP), whereas Pax2 expression is lost [12]–[14].
How the astrocytic network facilitates retinal vascular development remains incompletely understood. In spite of the important role of VEGF-A in vascular development [15]–[17], astrocyte specific disruption of VEGF-A expression did not interfere with retinal vascular growth, although vascular stability was compromised [18]. Thus, VEGF-A for retinal vascular development is presumably derived from non-astrocytic cells. However, VEGF-A extracellular distribution in developing retinas may be controlled by astrocyte-derived fibronectin and heparan sulfate [3], [4]. In addition, R-cadherin in retinal astrocytes is also important for retinal vascular development, which was demonstrated by blocking R-cadherin function with a neutralizing antibody [19].
A recent study found that HIF-1α deficiency in retinal neural tissues led to compromised development of both astrocytic and vascular networks [20]. In the present study, we compare contributions of HIF-1α and HIF-2α to the development of retinal astrocytic and vascular networks, with an emphasis on the role of HIF-2α in the astrocytic lineage. Selective Hif-2α disruption in Pax2+ astrocyte progenitor cells led to precocious and accelerated differentiation of Pax2+ progenitors into GFAP+ astrocytes, causing a shortage in the supply of Pax2+ progenitors and premature cessation of astrocyte development. Because HIF-2α protein undergoes oxygen dependent degradation, our findings suggest that retinal vascular development may modulate astrocyte development by regulating Hif-2α protein levels.
Materials and Methods
Mice
All animal procedures were approved by the Animal Care Committee at the University of Connecticut Health Center in compliance with Animal Welfare Assurance. Mice were housed with a 12 light/12 darkness cycle, and were maintained on normal chow. Mice were bred by natural mating, and the day when a litter was born was designated P0.
Floxed Hif-1α mice were originally produced by Randall Johnson's lab [21] and purchased from the Jackson laboratory in C57BL/6 (B6 from here on) strain background. These mice were crossed with CD1 and subsequently maintained in B6/CD1 mixed background. Floxed Hif-2α mice were produced in our own lab from B6/129 hybrid ES cells [22], and then backcrossed to B6 for 4 generations. At this point reproduction slowed down significantly, and a subgroup of the mice were crossed with CD1 females, leading to a population of floxed Hif-2α mice in mixed CD1 and B6 background at approximately 50%∶50% ratio. Two GFAPCre lines were purchased from the Jackson laboratory, including a line originally generated by Albee Messing (Jax stock number 004600) [23] and another line donated by Michael Sofroniew (line 77.6, Jax stock number 012887) [24]. GFAPCre mice originating from the Messing lab were supplied in FVB/N background. These mice were backcrossed into B6 for 4 generations, before they were crossed with CD1 females, resulting in mixed CD1/B6 background similar to that in floxed Hif-2α mice. The line 77.6 GFAPCre mice from the Sofroniew lab were received in B6 strain background, and were crossed into CD1 by one generation. The tdTomato mice carried a CAG promoter- loxP-Stop-loxP-tdTomato transgene targeted into the ubiquitously expressed Rosa26 locus [25], and were supplied in B6 strain background. These mice were crossed to CD1 by one generation before being crossed to GFAPCre mice. Tie2Cre was originally generated by Richard Flavell's group [26], and supplied by the Jackson lab (Jax stock number 004128). Detailed breeding information is summarized in Table S1.
Cre recombinases in Tie2Cre or GFAPCre mice were both constitutively active. The Rosa26CreERT2 mouse line was a gift from A. Joyner (New York University School of Medicine, New York, New York, USA) and was similar to a related mouse line described by Shebler et. al. [27], [28]. Rosa26CreERT2-encoded CreERT2 was activated by tamoxifen. Unless specifically indicated, neonatal mice were treated with tamoxifen by daily oral gavage from postnatal day 1 (P1) through P3 (40 mg/kg of body mass, with tamoxifen dissolved in corn oil). In some experiments, pregnant mothers were treated with tamoxifen by oral gavage at 17.5 days post coitum, followed by two more doses delivered to neonates at P1 and P2. In addition, some mice were treated with tamoxifen at P0 through P2. In all cases, pups with or without Rosa26CreERT2 were treated with tamoxifen in parallel.
Genotyping
Mouse ear punch (for weaned mice) and toe clips (for neonatal mice younger than P8) were used for DNA extraction. To determine deletion efficiency in the retina, P5 retinal tissues were used for DNA isolation and PCR analysis. Various alleles were identified using the following primer pairs: Hif-1α primers were as described previously [29]; Hif-2α primers: EPASloxF (AGTTCTGGCTCCTGCAAGAA) and EPASloxR (TTGCCAGAGGGGAGATGCTAAAATG), wt, 638 bp; floxed, 877 bp; deleted, 260 bp; Rosa26CreERT2 knock in allele was determined as described previously [27]; Tie2Cre transgene: Tie2CreF (CCGCCTGCTTCTGTGGTG) and Tie2CreR (GCCTGGCGATCCCTGAAC), 260 bp; GFAPCre transgene: GFAPCreF (ACTCCTTCATAAAGCCCTCG) and GFAPCreR (ATCACTCGTTGCATCGACCG), 230 bp. All PCR reactions were carried out under the following conditions: 94°C for 9 minutes to activate Taq DNA polymerase (Life Technologies), followed by 35 cycles at 55°C for 1 minute, 72°C for 4 minutes, and 94°C for 30 seconds.
Whole mount staining of neonatal retinas with isolectin B4 conjugated to Alexa fluor®-594(IB4-Alexa 594)
IB4 staining of neonatal retinas was carried out as described before [30]. Briefly, neonatal mice were euthanized by decapitation at indicated stages between P0 and P8, and eyes were isolated by enucleation. Isolated eyes were fixed with 4% paraformaldehyde (PFA) for 45 minutes at room temperature, following which retinas were dissected out. Retinas were cut by four incomplete radial incisions, leaving 4 pedals attached to one another at the center. Retinas were incubated at 4°C overnight with IB4-Alexa 594 (Life Technologies) at 1∶100 dilution in Retina Staining Buffer (RSB), which consisted of phosphate buffered saline (PBS), 1 mM CaCl2, 1 mM MgCl2, 1% Triton X100, and 1% BSA. Stained retinas were washed three times in RSB (1 hour each with rocking), and flat-mounted in 50% glycerol in PBS. Imaging was carried out by confocal microscopy.
Immunofluorescence (IF) staining of flat mount retinas
Retinas were prepared as described for IB4-Alexa 594 staining, and incubated with primary antibodies in RSB at 4°C overnight. Primary antibodies included rabbit anti-GFAP (1∶200, Life Technologies), rat anti-GFAP (2 µg/ml, Life Technologies), rabbit anti-Pax2 (1 µg/ml, Life Technologies), and goat anti- PDGFRα (1 µg/ml, R&D Systems). For Pax2 and GFAP double IF staining, rabbit anti-Pax2 and rat anti-GFAP were used. Following incubation with primary antibodies, retinas were washed, and incubated overnight with appropriate secondary antibodies including goat anti-rabbit IgG-Alexa fluor®-488 (1∶200, Life Technologies), donkey anti-rat IgG-Cy3 (2 µg/ml, Jackson ImmunoResearch, , West Grove, PA), and donkey anti-goat IgG-Alexa fluor®-488. Stained retinas were washed thoroughly, and mounted in 50% glycerol in PBS. Images were taken with a Zeiss LSM 510 confocal microscope.
Detection of proliferative and apoptotic astrocyte progenitors
For proliferation assay, neonatal mice were injected with 5-bromo-2-deoxyuridine (BrdU, 120 mg/kg of body mass; Roche Applied Science, Indianapolis, IN), and euthanized after 60 minutes. Retinas were fixed in 4% paraformaldehyde with the rest of the eyes still attached, and stored in 70% ethanol overnight. Thereafter, fixed retinas were dissected away from other eye structures, treated with 1% Triton X-100 in PBS for 30 minutes at room temperature, and then incubated in 2 mol/L HCl for 1 hour at 37°C. Processed retinas were double stained with mouse anti-BrdU antibody (BD Biosciences, San Jose, CA) and rabbit anti-Pax2, followed by goat anti-mouse IgG-Alexa fluor®-594 and goat anti-rabbit IgG-Alexa fluor®-488.
Apoptosis was monitored by IF staining with anti-active Caspase 3 (Abcam, Cambridge, MA). For positive control, neonatal mice were exposed to 75% oxygen at P7-P8 for 16 hours, returned to room air, and euthanized. Retinas from oxygen exposed mice were dissected, fixed, and stained with anti-active Caspase 3.
Immunofluorescence staining of retinal cryosections
Retinas prepared as above were embedded in OCT®, and cut at 6 µm. Sections were stained with rabbit anti-GFAP, rabbit anti-Pax2, or mouse anti-neurofilament (anti-NF, University of Iowa Developmental Studies Hybridoma Bank) in RSB for 2 hours. Afterwards, sections were washed, and stained with appropriate secondary antibodies, including goat anti-rabbit IgG-Alexa fluor®-488, or goat anti-mouse IgG-DyLight 488 (1∶200, Jackson ImmunoResearch). After washing and mounting, images were analyzed by confocal microscopy.
Preparation of nuclear protein extracts and Western blotting
Nuclear protein extract were prepared as described previously [31]. Briefly, neonatal mice were euthanized by decapitation, and retinas were isolated immediately in pre-chilled PBS, which was frequently replaced with additional aliquots of PBS precooled on ice. Nuclear protein extracts were prepared by homogenizing retinas in ice cold nuclear extraction buffer (10 mM HEPES-KOH (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, 0.2 mM deferoxamine (Sigma), 0.1% NP-40, and 1× complete protease inhibitor cocktail (Roche)). Nuclei were collected by centrifugation, and resuspended in ice cold re-suspension buffer containing 20 mM HEPES-KOH (pH 7.9), 420 mM NaCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol, 0.2 mM deferoxamine, 1× protease inhibitor cocktail, 0.2 mM phenylmethylsulfonyl fluoride, and 25% glycerol. The following antibodies were used for Western blotting: anti-HIF-1α (NB100-449, Novus Biologicals), anti-HIF-2α (NB100-132, Novus Biologicals), and anti-ß-actin (sc-1616; Santa Cruz Biotechnology).
Statistical analysis
Data were evaluated by two tailed Student's t-tests using Excel, and are presented as means ± standard error of means (SEM). All n values refer to number of mice. p<0.05 was considered significant.
Results
Differential but partially overlapping roles of HIF-1α and HIF-2α in retinal vascular development
To compare the roles of HIF-1α and HIF-2α, we generated Hif-1αf/f/Rosa26CreERT2 (f = floxed) and Hif-2αf/f/Rosa26CreERT2 mice. To activate CreERT2, neonatal mice were treated with tamoxifen by daily oral gavage at P1–P3. This procedure was previously shown to result in highly efficient deletion of floxed exons in a variety of tissues including the neonatal retina [27], [31]. In this study, we were also able to demonstrate significant deletion in retinal tissues (Figure S1).
At P8, the development of primary vascular plexus was examined by IB4 staining of retinas from mice treated with tamoxifen at P1–P3. Vascular development was indistinguishable between Hif-1αf/f and Hif-1αf/f/Rosa26CreERT2 mice (Figure 1A, D, G, and J), but was significantly reduced in Hif-2αf/f/Rosa26CreERT2 mice (Figure 1B, E, H, and K). The number of vascular branches was quantified in 0.2×0.5 mm2 areas midway between the optic nerve head and the periphery (Figure 1M). Hif-1αf/f and Hif-1αf/f/Rosa26CreERT2 mice both had similar number of vascular branches (104.3±7.4 and 95.2±8.7 per 0.1 mm2, respectively; n = 6, p = 0.44). By contrast, Hif-2αf/f/Rosa26CreERT2 mice had substantially reduced branching activities (102.1±5.2 in Hif-2αf/f mice, 49.3±11.1 in Hif-2αf/f/Rosa26CreERT2 mice, n = 7, p<0.01). When tamoxifen treatment was performed at earlier time points (starting at P0 or E17.5), vascular morphogenesis was further reduced in Hif-2αf/f/Rosa26CreERT2 mice (Figure S2).
Figure 1. Differential but partially overlapping roles of HIF-1α and HIF-2α in retinal vascular development.
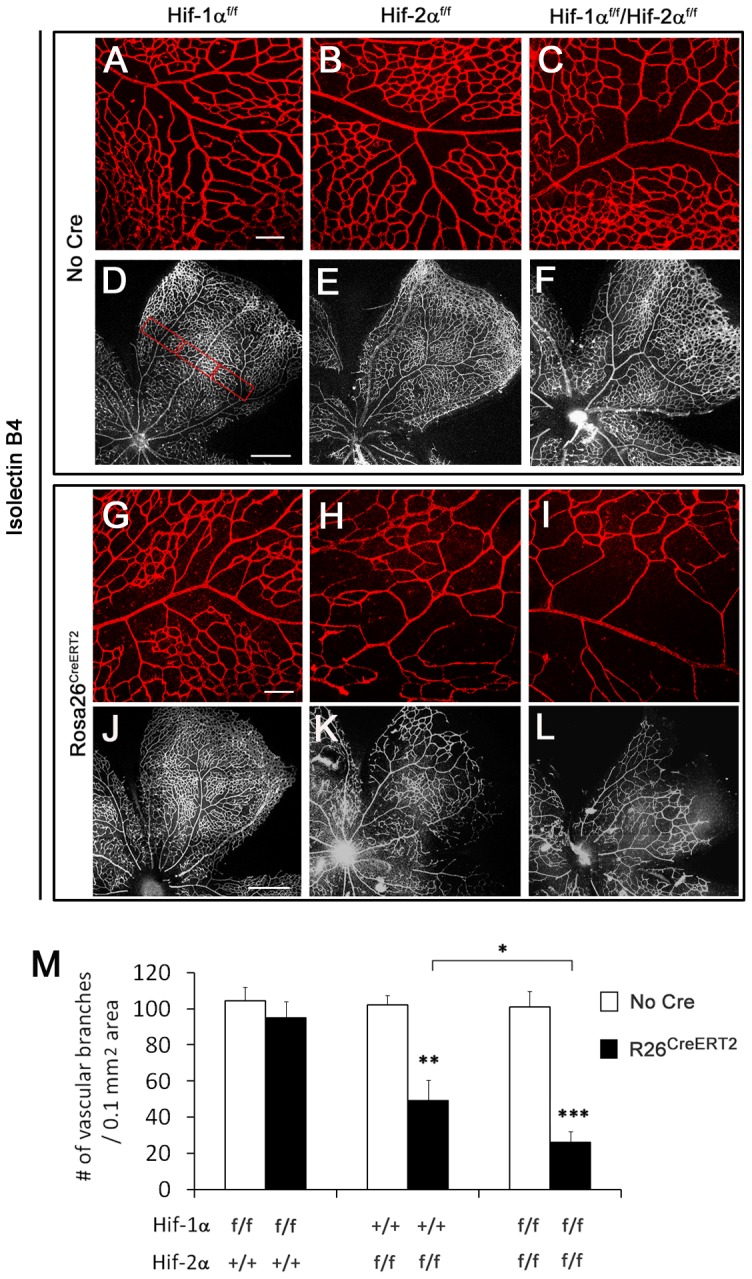
A to L. Confocal images of flat-mounted retinas stained with IB4-Alexa 594. A to C and G to I are representative images from areas midway between the optic nerve head and periphery; D to F and J to L each displays one of the 4 pedals of flat-mounted retinas. Images were tiled from multiple panels due to the size of retinas. Genotypes at the Hif-α and Rosa26 loci are indicated on top and to the left of the images, respectively. For example, panels G and J are Hif-1αf/f/Rosa26CreERT2. All mice were treated with tamoxifen by daily oral gavage at P1 through P3 (40 mg/kg in corn oil). At P8, retinas were dissected and stained with IB4-Alexa 594. Note that vascular development was reduced in H and K relative to G and J, respective, and further reduced in I and L. Hyaloid vessels were removed during dissection. Scale bars: A and G, 100 µm; D and J, 500 µm. M. Quantification of vascular branches. LoxP modifications at Hif-1α and Hif-2α loci are indicated below the bar graph, and presence or absence of Rosa26CreERT2 (R26CreERT2) is indicated by solid or open bars, respectively. Quantification was carried out with the assistance of NIH ImageJ. To average out local variations, vascular branches were counted in 3 rectangular areas (0.2×0.5 mm2) areas midway between the optic nerve head and the periphery, with an example shown in D. Where a single pedal was not cut wide enough to encompass all three rectangular areas, the third area was counted in an adjacent pedal. Average values from three areas were used as one data point for statistical analysis. n = 6. * p<0.05; ** p<0.01; *** p<0.001.
To determine if HIF-1α and HIF-2α might have any overlapping functions, we generated Hif-1αf/f/Hif-2αf/f/Rosa26CreERT2 mice. Vascular development in double disrupted mice was not only defective compared to floxed controls (Figure 1C, F, I, and L), but was also more severely compromised than in Hif-2αf/f/Rosa26CreERT2 mice (Figure 1H, I, K and L). While the number of vascular branches in Hif-2αf/f/Rosa26CreERT2 mice was about half of floxed controls, the corresponding value in Hif-1αf/f/Hif-2αf/f/Rosa26CreERT2 mice was further reduced to about 26.0% (Figure 1M).
Apparently normal retinal vascular development following Tie2Cre-mediated disruption of floxed Hif-1α or Hif-2α in endothelial cells
Next, we investigated if Hif-1α or Hif-2α disruption in endothelial cells might cause vascular deficiency. For this test, we used Tie2Cre which was well documented for its high efficiency in deleting floxed DNA sequences in endothelial and hematopoietic cells [26], [32]. For example, we showed in a previous study that Tie2Cre mediated disruption of floxed Vegfr-1 led to vascular defects identical to those in Vegfr-1 germline null embryos [30].
To directly test Cre activity in the context of retinal vascular development, we crossed Tie2Cre mice with tdTomato reporter mice, the latter of which carried a Cre-inducible tdTomato expression cassette integrated into the Rosa26 locus [25]. Tie2Cre specifically activated the expression of tdTomato in retinal vascular structures (Figure 2A and B), demonstrating that within the retina, Cre activity was most predominantly located in vascular endothelial cells. In Hif-1αf/f/Tie2Cre or Hif-2αf/f/Tie2Cre mice, retinal vascular patterns were indistinguishable from those in floxed mice (Figure 2C to F), suggesting that endothelial HIF-1α or HIF-2α was not essential for normal vascular development in neonatal retinas.
Figure 2. Apparently normal development of the primary layer of retinal vasculature in Hif-1αf/f/Tie2Cre and Hif-2αf/f/Tie2Cre mice.
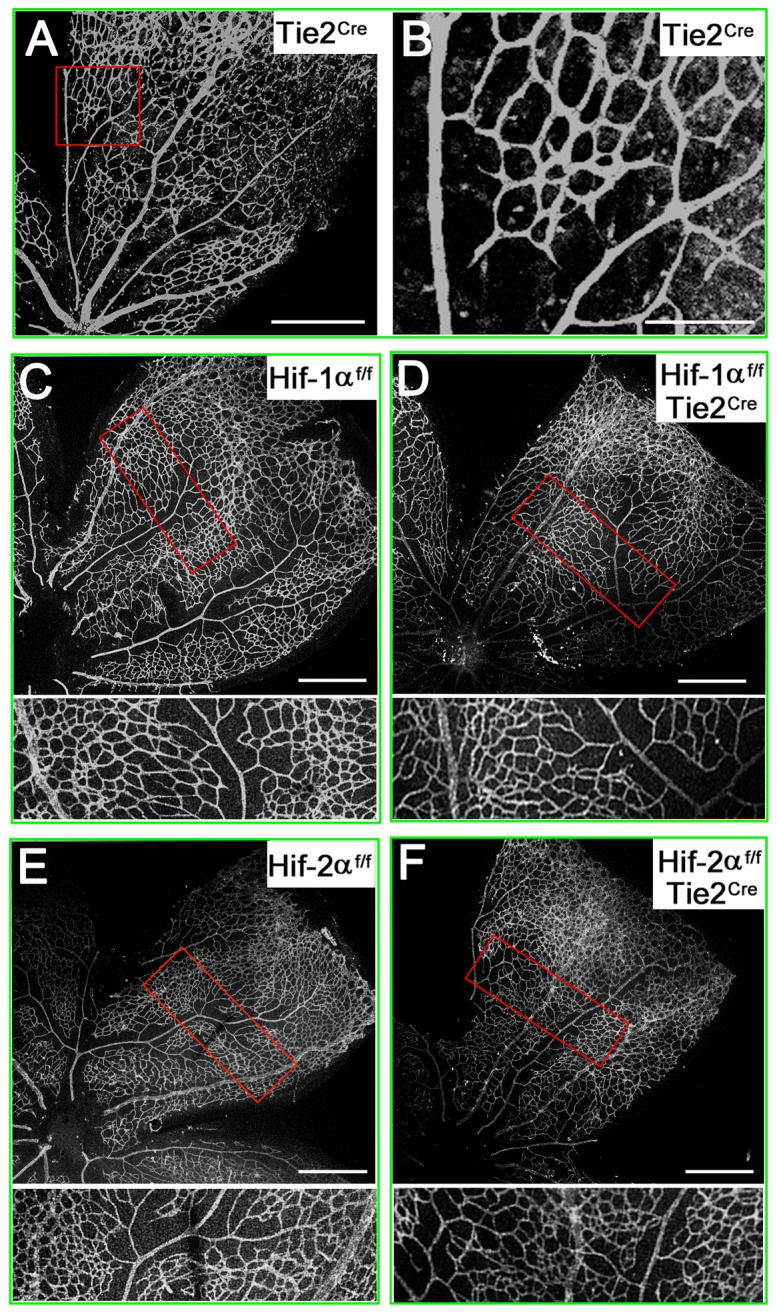
A and B. Confocal images of flat-mounted P7 retinas from tdTomato reporter mice carrying Tie2Cre transgene. B is expanded from the red rectangle in A. Clearly visible vascular patterns indicate that Tie2Cre is fully functional in retinal blood vessels. C to F. P8 retinas stained with IB4-Alexa 594. Areas in red rectangles are shown at higher magnifications below each source image. Images are representative of 4 to 6 mice in each group. Scale bars are 400 µm in A, 125 µm in B, and 500 µm in C–F.
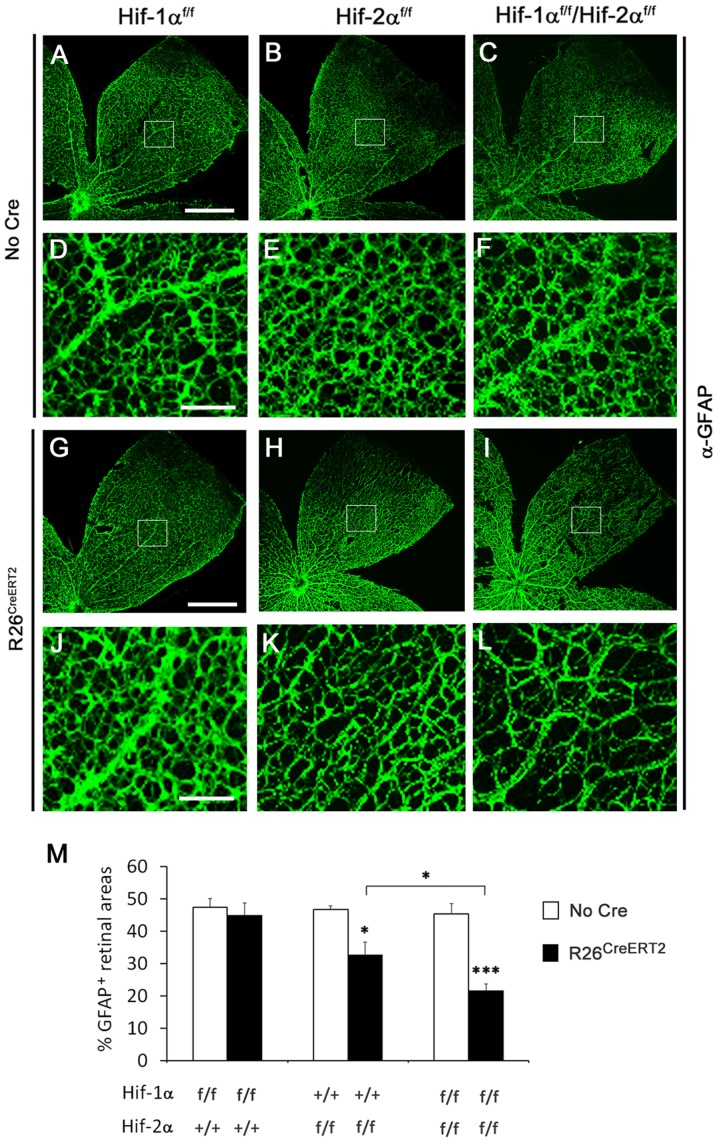
Reduced development of the astrocytic network in association with HIF-2α deficiency
The above findings suggested that the requirement for HIF-α proteins in retinal vascular development might reside in non-endothelial cells. Thus, we examined the development of the retinal astrocytic network by anti-GFAP IF staining in whole mount retinas. At P8, the morphology of astrocytic networks was essentially the same between Hif-1αf/f and Hif-1αf/f/Rosa26CreERT2 mice (Figure 3A, D, G, J). However, Hif-2αf/f/Rosa26CreERT2 mice displayed substantially reduced astrocyte development compared to Hif-2αf/f mice (Figure 3B, E, H and K). In Hif-2αf/f/Rosa26CreERT2 mice, retinal tissues were less densely populated with GFAP+ astrocytes, and individual astrocyte branches in the network appeared thinner than their counterparts in Hif-2αf/f mice. Interestingly, astrocyte development was further reduced in Hif-1αf/f/Hif-2αf/f/Rosa26CreERT2 mice (Figure 3C, F, I, and L).
Figure 3. Reduced retinal astrocyte development in Hif-2αf/f/Rosa26CreERT2 mice.
A to L. Confocal images showing retinal astrocytic networks of P8 neonatal mice. All mice were treated with tamoxifen by daily oral gavage at P1 through P3. At P8, retinas were dissected, fixed, and stained with rabbit anti-GFAP followed by goat anti-rabbit IgG-Alexa 488. Boxed areas in A–C and G–I are shown at higher magnifications below each source image. Scale bars are 500 µm in A and G and 100 µm in D and J. M. Percentage of retinal area occupied by GFAP+ cells. Quantification was carried out midway between the optic nerve and periphery (white boxes). Equivalent areas in three different pedals were counted for each retina, and the average value was used as one data point. Hif-1a and Hif-2a alleles are indicated below each bar; presence of R26CreERT2 is indicated by solid bars. n = 6; * p<0.05; *** p<0.001.
The development of astrocytic networks was quantified by calculating the percentage of GFAP+ tissues in areas midway between the optic nerve and the periphery (Figure 3M). In Hif-1αf/f and Hif-1αf/f/Rosa26CreERT2 mice, GFAP+ areas occupied 47.4±2.8% and 44.9±3.8% of total areas, respectively (n = 6 per group, p = 0.61). In Hif-2αf/f and Hif-2αf/f/Rosa26CreERT2 mice, the corresponding values were 46.7±1.2% and 32.7±3.9% (n = 7 per group, p<0.05). In Hif-1αf/f/Hif-2αf/f/Rosa26CreERT2 mice, GFAP+ area was down to 21.6±2.1%, which was not only significantly lower than 45.4±3.1% in Hif-1αf/f/Hif-2αf/f mice (n = 7 per group, p<0.001), but also lower than in Hif-2αf/f/Rosa26CreERT2 mice (p<0.05).
Requirement of astrocyte-derived HIF-2α for vascular development
To determine if HIF-2α was required in astrocytes, we decided to disrupt floxed Hif-2α with GFAP promoter-driven Cre. Several different GFAPCre lines had been reported previously [23], [24], [33], two of which were tested in this study, including line 77.6 from the Sofroniew lab and the line from the Messing lab. To examine Cre activity in neonatal retinas, these Cre lines were crossed with tdTomato reporter mice. Unexpectedly, despite reported expression in astrocytes in the brain, line 77.6 failed to activate any tdTomato expression in the retina (data not shown). In contrast, the line from the Messing lab activated tdTomato expression predominantly in Pax2+ astrocyte progenitors and GFAP+ astrocytes at P0, P3 and P5 (Figure S3 and Figure S4). Since we have not investigated GFAPCre activities at developmental stages beyond the focus of this study, our data do not rule out likely GFAPCre activity in other cell types in older mice.
We generated Hif-1αf/f/GFAPCre and Hif-2αf/f/GFAPCre mice using GFAPCre mice originated from the Messing lab, and visualized retinal vascular structures at P8 by whole mount staining with IB4-Alexa 594. As shown in Figure 4A to C, vascular patterns were similar among Hif-1αf/f , Hif-1αf/f/GFAPCre and GFAPCre mice. In sharp contrast, Hif-2αf/f/GFAPCre mice displayed drastically reduced vascular development relative to floxed controls (Figure 4D to F). Among 22 Hif-2αf/f/GFAPCre mice examined at P8, only 2 had remnant amounts of blood vessels as shown in Figure 4D, but the remaining 20 were completely devoid of blood vessels (Figure 5F). While the primary retinal vascular network was absent in most of Hif-2αf/f/GFAPCre mice, hyaloid vascular structures were abundantly present (Figure S5).
Figure 4. Dramatically reduced retinal vascular development in Hif-2αf/f/GFAPCre mice.
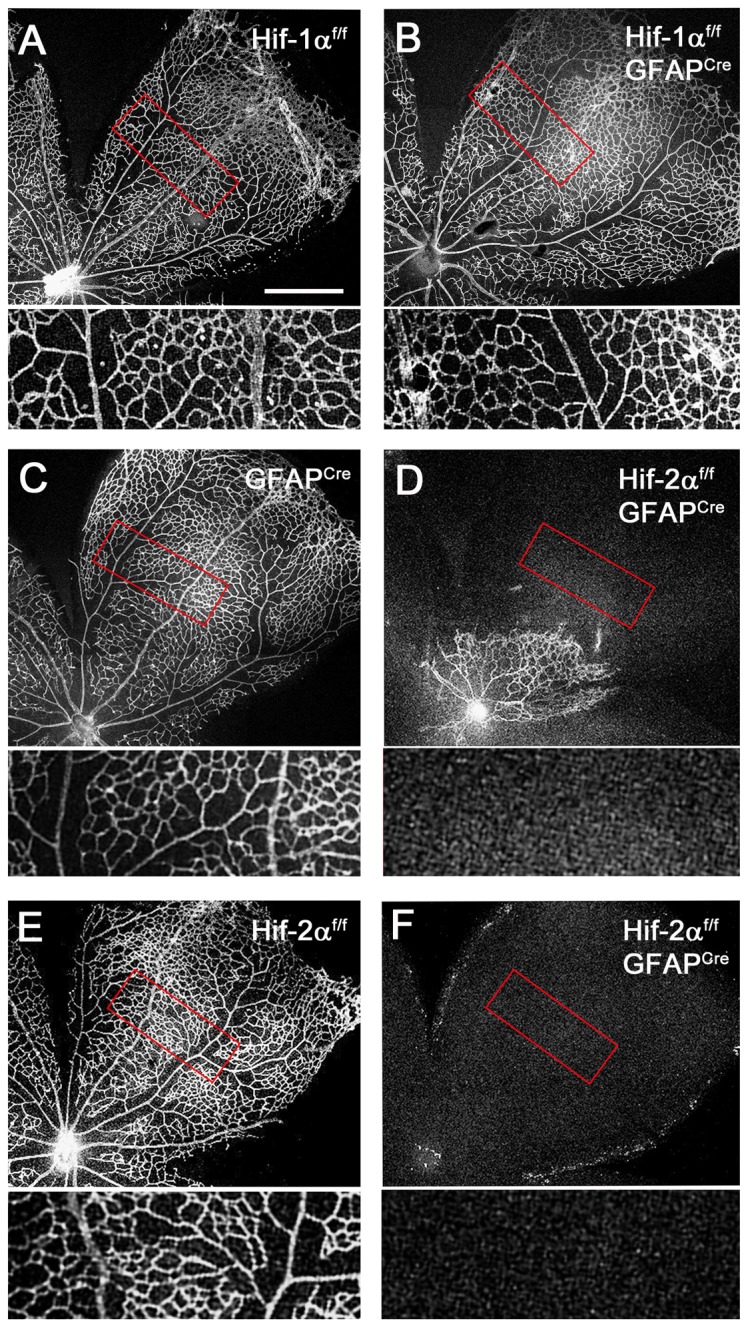
All retinas were dissected at P8, fixed and flat-mounted, and stained with IB4-Alexa 594. Development of the primary retinal vascular beds was analyzed by confocal microscopy. A and B. Hif-1αf/f and Hif-1αf/f/GFAPCre mice. C to F. GFAPCre (C), Hif-2αf/f (E), and Hif-2αf/f/GFAPCre (D and F) mice. Areas in red rectangles were expanded and shown below each main image. Out of 22 Hif-2αf/f/GFAPCre mice analyzed, 2 had remnant amounts of blood vessels, as shown in D, whereas the rest were completely devoid of any vascular development (F). Hyaloid vessels were removed during dissection. Scale bar represents 500 µm, and all main images are in the same magnification scale.
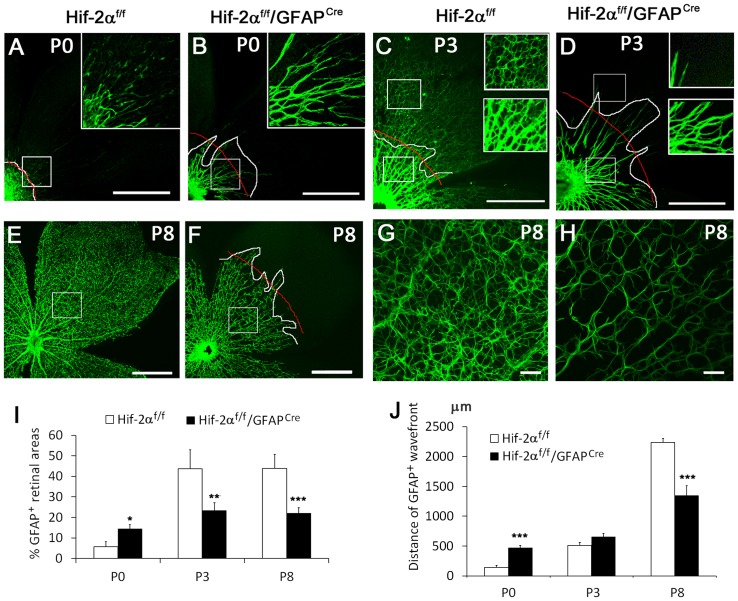
Figure 5. Precocious but tapered development of the astrocytic network in Hif-2αf/f/GFAPCre mice.
Astrocyte development was examined by anti-GFAP IF staining. A and B. Anti-GFAP staining at P0. In A, GFAP+ cells are mostly limited to the optic nerve head (lower left). In Hif-2αf/f/GFAPCre mice, GFAP+ structures extended much further. White lines demarcate the borderline between strongly GFAP+ and the rest of retinal areas. Red lines mark the approximate average position of the white line. Boxed areas are shown in higher magnifications in the insets. C and D. Anti-GFAP staining at P3. Upper and lower insets are expanded from corresponding boxes. E to H. Anti-GFAP stained retinas at P8 shown at low (E and F) and high (G and H) magnifications. Scale bars, 500 µm in A to F, 50 µm in G and H. I. Percentage (%) of GFAP+ areas. Quantifications were carried out in white boxes in A, B, E and F, and lower boxes in C and D. Three such areas were quantified per mouse and average values were used as one data point. J. Quantification of GFAP+ wavefronts to the optic nerve, measured as distances between red curves and optic nerves. n = 5. * p<0.05, ** p<0.01, *** p<0.001.
Precocious but tapered retinal astrocyte differentiation in Hif-2αf/f/GFAPCre mice
Data presented above strongly pointed to the possibility that retinal astrocyte development might be defective in Hif-2αf/f/GFAPCre mice. Unexpectedly, retinas from Hif-2αf/f/GFAPCre mice exhibited significantly more active astrocyte differentiation at P0 (Figure 5A, B, I, and J). Strong GFAP+ staining was mostly limited to the optic nerve head in floxed mice but extended much further in Hif-2αf/f/GFAPCre mice. By P3, the development of astrocytic networks started to lag behind in Hif-2αf/f/GFAPCre mice (Figure 5C, D, I and J). While strongly GFAP+ structures extended similar distances from optic nerves in Hif-2αf/f and Hif-2αf/f/GFAPCre mice, astrocytes in the latter failed to form complex network organization and occupied less percentage of retinal areas. Further away from the optic nerve head, weakly GFAP+ structures were abundantly present in Hif-2αf/f but not Hif-2αf/f/GFAPCre mice (Figure 5C and D, upper rectangles and insets).
By P8, Hif-2αf/f/GFAPCre mice were completely overtaken by floxed controls (Figure 5E to H). Differences in Hif-2αf/f and Hif-2αf/f/GFAPCre mice were manifested in three ways. First, whereas GFAP+ cells had spread out over the entire retinal area in Hif-2αf/f pups, they only reached approximately midway in Hif-2αf/f/GFAPCre mice (Figure 5E and F). Second, within GFAP+ territories, Hif-2αf/f/GFAPCre mice were less densely populated with astrocytes (Figure 5G, H, I and J). Third, astrocytes in Hif-2αf/f/GFAPCre mice were much thinner and had fewer branches (Figure 5G and H). In contrast to the dramatic effects of GFAPCre-mediated Hif-2α disruption on astrocyte development, little difference was found between Hif-1αf/f and Hif-1αf/f/GFAPCre mice. As shown in Figure S6A and B, anti-GFAP staining patterns at P8 were indistinguishable between floxed and Hif-1α disrupted mice.
We also evaluated retinal astrocyte development by anti-PDGFRα staining. At P0, PDGFRα positive astrocyte networks expanded towards the retinal periphery by similar distances in Hif-2αf/f and Hif-2αf/f/GFAPCre mice (Figure 6A, B and E). Within PDGFRα+ territories however, the percentage of area occupied by PDGFRα+ structures is much lower in Hif-2αf/f/GFAPCre mice (Figure 6A, B and F). At P3, PDGFRα+ astrocytes covered essentially the entire retinal inner surface in Hif-2αf/f mice, but were limited to the more central half in Hif-2αf/f/GFAPCre mice (Figure 6C, D and E). Within PDGFRα+ retinal areas, percentage area occupied by PDGFRα+ astrocytes was also significant lower in Hif-2αf/f/GFAPCre mice (Figure 6C, D and F).
Figure 6. Reduced number of PDGFRα+ astrocytes in Hif-2αf/f/GFAPCre mice.
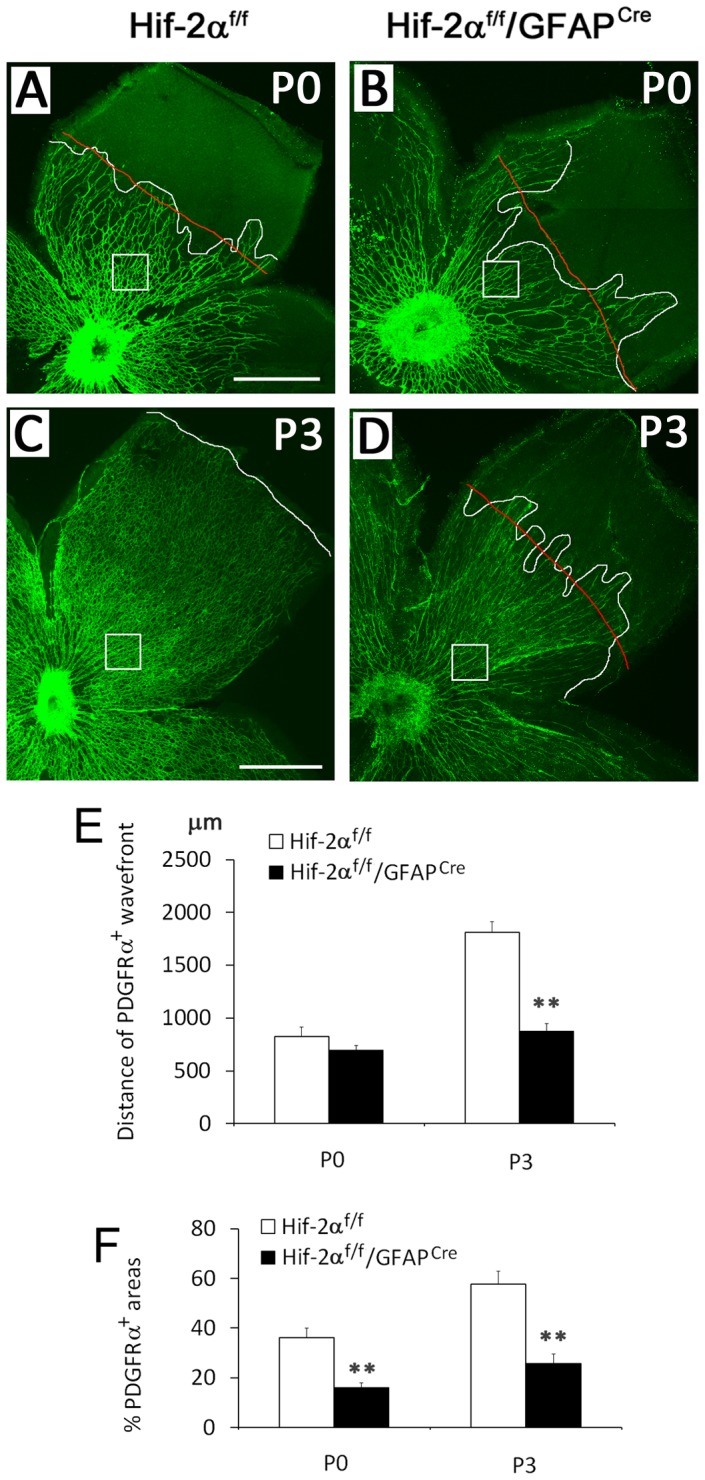
A to D. Retinas dissected at P0 and P3 were subject to anti-PDGFRα IF staining. Wavefronts of PDGFRα+ astrocytes are marked by white lines. Red lines represent the approximate average positions of the zigzag white lines. In C, no red line is provided because the front of PDGFRα+ astrocyte network was relatively even. Areas indicated by white boxes were quantified for percentage of PDGFRα+ tissues. E. Distance from optic nerve head to the front of PDGFRα+ areas (white line in C, or red lines in A, B, and D). F. Percentage of PDGFRα+ retinal areas. Quantifications were carried for areas indicated by white boxes, taking the average of three such areas from the same mouse as one data point. Scale bars are 500 µm. n = 4. ** p<0.01.
Depletion of Pax2+ astrocyte progenitors by GFAPCre-mediated Hif-2α disruption
To further investigate retinal astrocyte differentiation in Hif-2αf/f/GFAPCre mice, we analyzed the behavior of Pax2+ cells, which represent retinal astrocyte progenitors. At P0, the presence of Pax2+ cells was significantly reduced in Hif-2αf/f/GFAPCre retinas (Figure 7A, B, and K). This difference became more dramatic by P4 (Figure 7C, D and K). We reasoned that increased astrocyte differentiation at early stages might have occurred at the expense of Pax2+ progenitor population growth, happening too early and too fast, which resulted in the depletion of astrocyte progenitors and immature astrocytes. To test this hypothesis, we directly compared the development of Pax2+ and GFAP+ cells by anti-Pax2 and anti-GFAP double IF staining at P0. In Hif-2αf/f mice, GFAP+ signals were mostly limited to the optic nerve head area, but Pax2+ cells were abundantly present both near and further beyond the optic nerve head (Figure 7E and F). Pax2+ and GFAP+ domains did not colocalized, but instead formed a complementary pattern (Figure 7G), demonstrating that Pax2+ population was expanding beyond the optic nerve head without significant astrocyte differentiation at this stage of development. In Hif-2αf/f/GFAPCre littermates, retinas had already developed numerous GFAP+ sprouts but were less densely populated with Pax2+cells than in Hif-2αf/f controls (Figure 7H and I). Merged images also revealed that both Pax2+ and GFAP+ cells were present off the optic nerve head in Hif-2αf/f/GFAPCre mice (Figure 7J), suggesting that Pax2+ cells were quickly differentiating into GFAP+ mature astrocytes.
Figure 7. Depletion of Pax2+ astrocyte progenitors in Hif-2αf/f/GFAPCre mice.
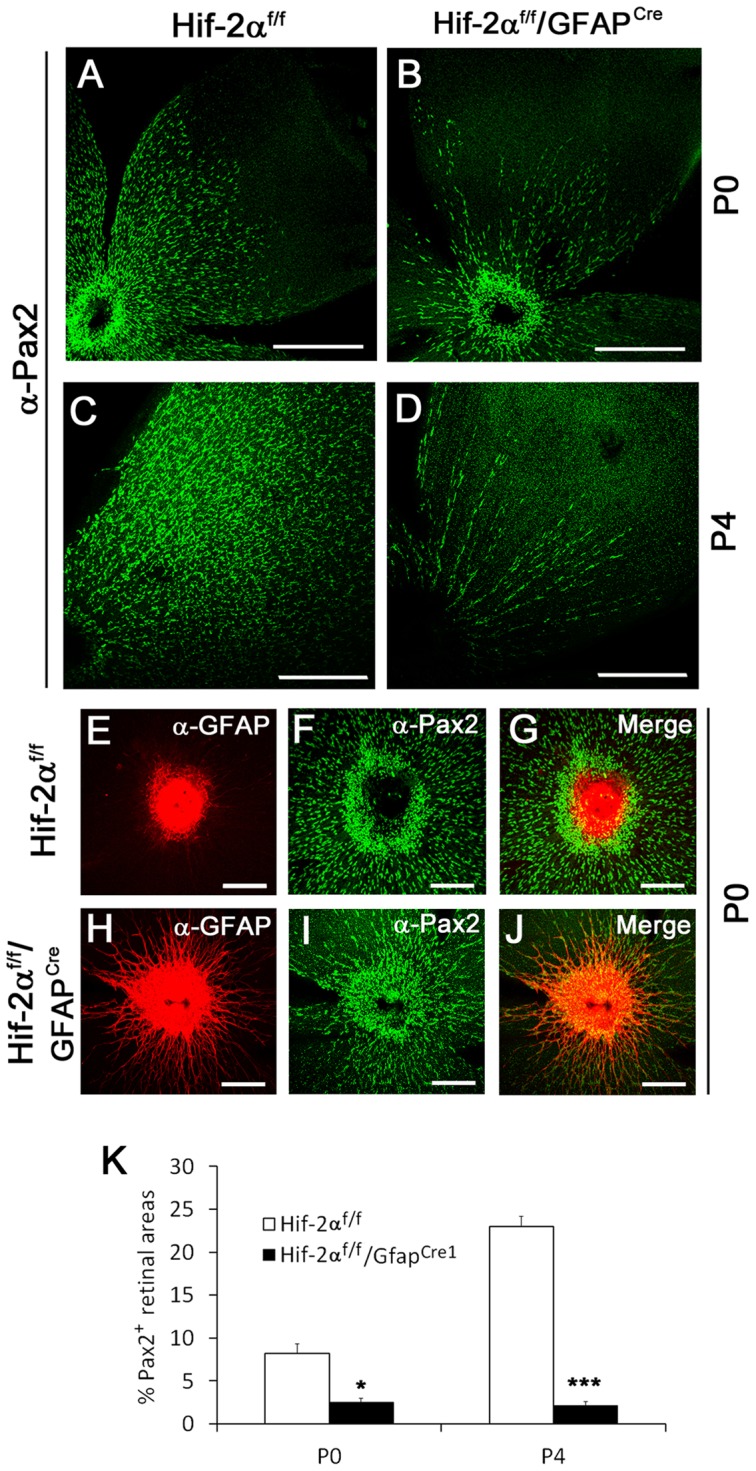
A to D. Anti-Pax2 IF staining of retinas at P0 (A and B) and P4 (C and D). The abundance of Pax2+ cells was dramatically reduced in Hif-2αf/f/GFAPCre mice at both P0 and P4. Scale bars are 500 µm. E to J. Double IF staining of P0 retinas with rabbit anti-Pax2 and rat anti-GFAP, followed by goat anti-rabbit IgG-Alexa 488 and donkey anti-rat IgG-Cy3. In Hif-2αf/f retinas, Pax2+ (green) are abundantly present in the vicinity of strongly GFAP+ (red) optic nerve head, but mature GFAP+ astrocytes are virtually absent. In Hif-2αf/f/GFAPCre mice, Pax2+ cells less abundantly present off the optic nerve, accompanied by many GFAP+ cells. Scale bars in E to J are 200 µm. K. Percentage (%) of retinal areas occupied by Pax2+ cells. n = 5. * p<0.05, *** p<0.001.
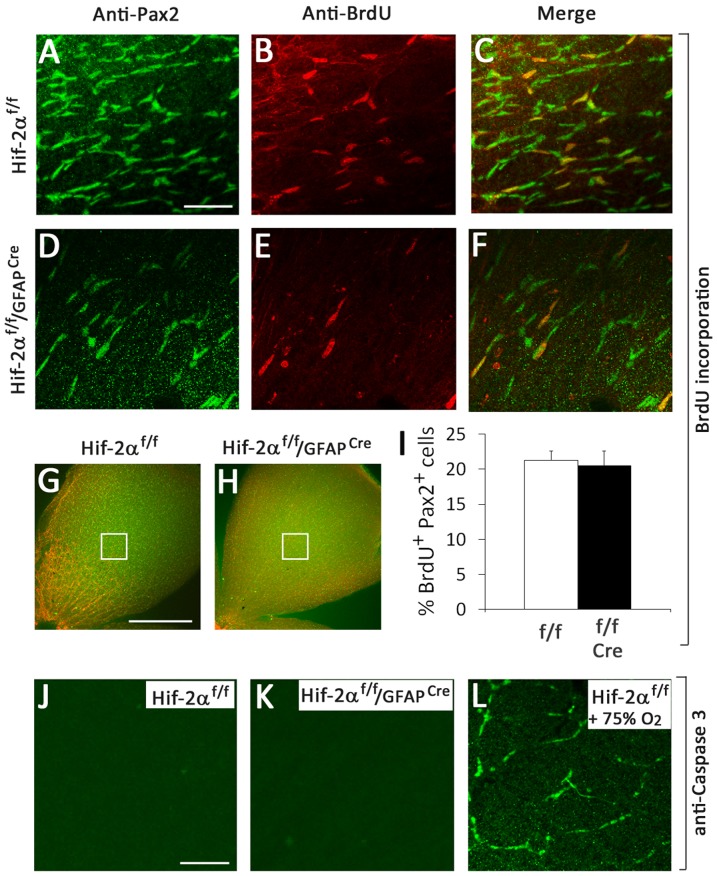
We also assessed proliferation of Pax2+ cells at P3. While Hif-2αf/f/GFAPCre mice contained less abundant Pax2+ cells, the percentages of BrdU-labeled Pax2+ cells did not differ between Hif-2αf/f and Hif-2αf/f/GFAPCre mice (Figure 8A–I). Apoptotic cells were not detectable by anti-active Caspase 3 staining in both Hif-2αf/f and Hif-2αf/f/GFAPCre mice (Figure 8J and K). As a positive control, we performed the same analysis in retinas from hyperoxia treated mice. Apoptotic cells were readily detectable by anti-active Caspase 3 staining in the control (Figure 8L). Based on these findings, we conclude that loss of Pax2+ cells in Hif-2αf/f/GFAPCre retinas was unlikely a result of decreased proliferation or increased apoptosis.
Figure 8. Proliferation and apoptosis assays.
A to H. Proliferation assays. Mice were injected with BrdU at P3, and retinas were dissected after an hour. BrdU incorporation in astrocyte progenitors was detected by anti-BrdU and anti-Pax2 double IF staining. A to F show representative images from areas indicated in G and H. Both Pax2+ and BrdU+ cells were counted with the assistance of NIH ImageJ program. I. Proliferation index was calculated as % of Pax2+ cells that were also BrdU+. Cre refers to GFAPCre. n = 4. No significant difference was found between Hif-2αf/f and Hif-2αf/f/GFAPCre mice. J to L. Apoptosis assay by anti-active Caspase 3 IF staining. J and K are P3 retinas. No apoptotic cells were detected in either Hif-2αf/f or Hif-2αf/f/GFAPCre mice. L is from mice treated with 75% oxygen for 16 hours between P7 and P8. The same anti-active Caspase 3 staining procedure detected large numbers of apoptotic cells in oxygen-treated mice. Images are representative of data from 4 mice in each group. Scale bars are 50 µm for A to F, 500 µm for G and H, and 50 µm for J to L.
Discussion
Roles of HIF-α proteins in the development of retinal primary vascular plexus
HIF-1α and HIF-2α have well known roles in angiogenesis in a variety of tissues including embryos, ischemic tissues, and tumors [21], [34]–[41]. In the retina, both HIF-1α and HIF-2α are linked to pathological neoangiogenesis [42]–[44], and HIF-1α in peripheral retinal tissues mediates the development of the intermediate plexus [45]. In this study, global but not endothelial/hematopoietic HIF-2α deficiency led to diminished retinal vascular development. The lack of an essential role for HIF-2α in endothelial cells was not completely unexpected, because mice used in this study were in mixed CD1/B6 background whereas endothelial role of HIF-2α is mostly limited to the 129 strain [35], [36], [46].
HIF-2α deficiency in the astrocytic lineage led to severe vascular defects. This finding contrasts sharply to normal vascular development a previous study which used a different GFAPCre line to disrupt Hif-2a [44]. In the study described here, a GFAPCre line originally generated in the Messing lab was used [23], which robustly activated the expression of Cre-inducible tdTomato reporter in astrocyte progenitors as early as P0. We presume that early expression of GFAPCre in astrocyte progenitors may be responsible for the phenotypes presented in this manuscript.
Regulation of retinal astrocyte development by HIF-2a
In Hif-2αf/f/GFAPCre mice, astrocytic templates were only partially developed, and the morphology of HIF-2α deficient astrocytes was abnormally thin and elongated. These defects were associated with almost complete absence of retinal vascular development, suggesting that HIF-2α deficiency compromised not only the abundance but also the function of astrocytes. While the co-existence of vascular and astrocytic defects echoes previous findings that the development of retinal vasculature depends on the astrocytic template [2]–[4], a role of HIF-2α in retinal astrocyte differentiation may have further implications on the relationship between retinal astrocytic and vascular development. Since the stability of HIF-2α is sensitively regulated by prolyl hydroxylase domain proteins in an oxygen dependent manner [47]–[50], our findings raise the possibility that regulation of astrocytic and vascular development may be a two way process. In addition to the role of astrocytic templates in regulating retinal vascular growth, their own development may be regulated by oxygen diffusing out of retinal blood vessels. The defects in Hif-2αf/f/GFAPCre mice may reflect the exaggeration of an inherently important mechanism.
Interpretation of retinal astrocytic phenotypes in Hif-2αf/f/GFAPCre mice
Despite reduced astrocyte abundance in Hif-2αf/f/GFAPCre retinas after P3, astrocyte differentiation from their progenitors was initially increased instead of being decreased, proliferation index of astrocyte progenitors was not reduced, and apoptosis was not increased. These apparently paradoxical phenomena may be understood by proposing precocious and accelerated differentiation of astrocyte progenitors. Because mature astrocytes are non-proliferative, normal development of the astrocytic network depends on the proliferation of astrocyte progenitors and immature astrocytes. If progenitor cells differentiate into mature astrocytes too fast, the supply of the progenitor stock may be depleted because progenitor cells are deprived of the time needed for proliferation. Eventually, dwindling supply of progenitor cells may interrupt astrocyte development. In support of this viewpoint, Hif-2αf/f/GFAPCre mice indeed contained diminished numbers of Pax2+ astrocyte progenitors as soon as mice were born. Since Pax2 and PDGFRα are co-expressed in immature astrocytes [7], it is not surprising that the number of PDGFRα+ cells are similar reduced.
The failure of HIF-2α deficient astrocyte network to expand all the way to retinal periphery might also suggest defects in migration. However, this phenotype can be also explained by accelerated differentiation. As retinal development proceeds, the expansion of mature astrocyte network may depend on in situ differentiation from progenitors seeded at progressively more peripheral positions, rather than migration of fully differentiated astrocytes towards the periphery. Indeed, as early as P3, astrocyte progenitors and immature astrocytes have already spread out to essentially the entire inner surface in wild-type retinas (Figure S7). In Hif-2αf/f/GFAPCre mice, the available astrocyte progenitors were enough to cover only the more central part of the retinas, thus precluding astrocyte differentiation at more peripheral positions.
The model
Our data led to a model schematically illustrated in Figure 9, which states that HIF-2α regulates retinal astrocyte development by guarding the rate of astrocyte differentiation from immature progenitors. This function is important because astrocyte progenitors are proliferative whereas mature astrocytes are not. Without HIF-2α, accelerated astrocyte differentiation depletes astrocyte progenitors by disallowing them the time needed for proliferation. As a result, retinal astrocyte development ceases prematurely due to the lack of astrocyte progenitors. It should be pointed out that the physiological HIF-2α level in wild-type retinas is apparently insufficient to completely inhibit astrocyte differentiation, but maintains a balance between progenitor population growth and astrocyte maturation.
Figure 9. Model for HIF-2α regulated retinal astrocyte differentiation.
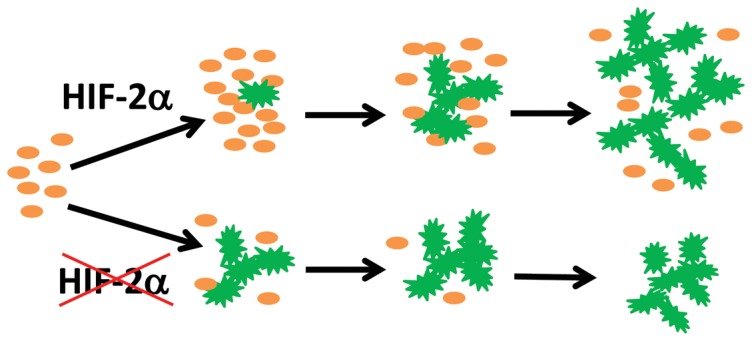
In wild-type mice, physiological level of HIF-2α may partially suppress differentiation of mature astrocytes (green stars) from their progenitors (orange ovals). Thus, while a proportion of astrocyte progenitors differentiate into non-proliferative mature astrocytes, the rest may undergo active proliferation to replenish progenitor populations. HIF-2α deficiency disturbs the balance between progenitor proliferation and astrocyte differentiation. Accelerated astrocyte differentiation may cause rapid loss of astrocyte progenitors due to inadequate time for proliferation, leading to unsustainable astrocyte development.
Supporting Information
Disruption of Hif-1α and Hif-2α . Pups were obtained by crossing Hif-1αf/f/Rosa26CreERT2 males with Hif-1αf/f females, or Hif-2αf/f/Rosa26CreERT2 males with Hif-2αf/f females. At P1–P3, pups were treated with tamoxifen by daily oral gavage. At P5, pups were euthanized, and retinas were dissected. Deletion of floxed Hif-1α (A) and Hif-2α (B) in retinal tissues was assessed by PCR of retinal DNA extracts. Floxed Hif-1α, 260 bp, deleted allele, 270 bp; floxed Hif-2α allele, 877 bp, deleted allele, 260 bp. HIF-1α and HIF-2α protein levels were determined by anti-HIF-1α (C) or anti-HIF-2α (D) Western blotting of retinal nuclear protein extracts.
(TIF)
Induction of more severe vascular defects in Hif-2αf/f/Rosa26CreERT2 mice by tamoxifen treatment at earlier time points. A and B. Hif-2αf/f and Hif-2αf/f/Rosa26CreERT2 neonatal mice were treated with tamoxifen at P0 through P2. C and D. Pregnant Hif-2αf/f females mated with Hif-2αf/f/Rosa26CreERT2 males were treated with a single dose of tamoxifen at 17.5 d.p.c.. Following birth, neonatal mice were treated with two more doses at P1 and P2. All retinas were stained with IB4 -Alexa 594 at P8. n = 3. Scales bar represents 50 µm.
(TIF)
GFAPCre activity in retinal astrocytes. GFAPCre mice were crossed with transgenic mice carrying a CAG promoter- loxP-Stop-loxP-tdTomato transgene targeted into the ubiquitously expressed Rosa26 locus. A to D. Co-localization of tdTomato expression with GFAP+ astrocytes. Retinas were dissected from neonatal mice at P5, and cryosections were cut at 6 µm. Sections were stained with rabbit anti-GFAP and anti-Rabbit IgG-Alexa 488, and analyzed by confocal imaging for tdTomato expression (A) or GFAP+ cells (B). Merged images are shown in C and D at different magnifications. tdTomato expression and GFAP+ cells colocalized at the inner surface of retinal tissues. E to H. Confocal images of tdTomato expression and anti-NF (neurofilament) immunofluorescence staining (green). Cryosections were prepared as in A to D, but stained with mouse anti-NF followed by goat anti-mouse IgG-DyLight 488. It is evident that tdTomato expression does not colocalize with NF+ signals. Scales bars, A–C and E to G, 100 µm; D and H, 50 µm.
(TIF)
GFAPCre activity in astrocyte progenitors. GFAPCre and Cre-inducible tdTomato transgenic mice were crossed, and pups carrying both transgenes were subject to anti-Pax2 or anti-GFAP IF staining at P0 and P3, and visualized by confocal imaging. The vast majority of tdTomato positive cells were also Pax2+ and GFAP+, demonstrating early onset of Cre activity in retinal astrocyte progenitors. n = 3. All images are at the same magnification. Scale bar represents 50 µm.
(TIF)
Hyaloid vessels in Hif-2α f/f/GFAPCre mice. Retinas were dissected at P8, care being taken to preserve hyaloid vessels. A and B. IB4-Alexa 594-stained retinas. C and D. Higher magnifications of the top right quarter from A and B, respectively. In C, white arrows point to main arterioles; green arrows indicate large branches between arterioles. Since every main arteriole has just one accompanying venules in normal retinas, additional large branches may be hyaloid vessels. Hyaloid vessels are more prominently present in Hif-2α f/f/GFAPCre mice, presumably to compensate for the loss of retinal blood vessels. n = 3.
(TIF)
Apparently normal astrocyte development in Hif-1αf/f/GFAPCre mice. At P8, retinas were dissected from Hif-1αf/f (A) and Hif-1αf/f/GFAPCre (B) mice, fixed, and stained by rabbit anti-GFAP followed by goat anti-rabbit IgG-Alexa 488. Data shown are representative confocal images from 4 mice in each genotype. Scales bars are 100 µm. Images are representative of data from 3 mice per group.
(TIF)
Astrocyte progenitors and immature astrocytes in P3 retinas. A to C are confocal images of wild-type (Hif-2αf/f) retinas stained with indicated antibodies at P3. Boxed areas are expanded and shown in D to F. Images in D to F were brightened to show more details at the periphery. These data confirm that by P3, astrocyte progenitors and immature astrocytes are already present throughout the retina. Images are representative of at least 3 mice each. Scales bars are 500 µm for A–C, and 100 µm for D to F.
(TIF)
Breeding strategy and strain background of relevant mouse lines.
(DOCX)
Funding Statement
The work was supported by NIH grant 5R01EY019721. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stone J, Dreher Z (1987) Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol 255: 35–49. [DOI] [PubMed] [Google Scholar]
- 2. Miyawaki T, Uemura A, Dezawa M, Yu RT, Ide C, et al. (2004) Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte development in the developing retina. J Neurosci 24: 8124–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uemura A, Kusuhara S, Wiegand SJ, Yu RT, Nishikawa S (2006) Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J Clin Invest 116: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenzel D, Lundkvist A, Sauvaget D, Busse M, Graupera M, et al. (2011) Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development 138: 4451–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu Y, Hughes S, Chan-Ling T (2001) Differentiation and migration of astrocyte precursor cells and astrocytes in human fetal retina: relevance to optic nerve coloboma. FASEB J 15: 2013–2015. [DOI] [PubMed] [Google Scholar]
- 6. Mi H, Barres BA (1999) Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J Neurosci 19: 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mudhar HS, Pollock RA, Wang C, Stiles CD, Richardson WD (1993) PDGF and its receptors in the developing rodent retina and optic nerve. Development 118: 539–552. [DOI] [PubMed] [Google Scholar]
- 8. Fruttiger M, Calver AR, Kruger WH, Mudhar HS, Michalovich D, et al. (1996) PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron 17: 1117–1131. [DOI] [PubMed] [Google Scholar]
- 9. West H, Richardson WD, Fruttiger M (2005) Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development 132: 1855–1862. [DOI] [PubMed] [Google Scholar]
- 10. Mi H, Haeberle H, Barres BA (2001) Induction of astrocyte differentiation by endothelial cells. J Neurosci 21: 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kubota Y, Hirashima M, Kishi K, Stewart CL, Suda T (2008) Leukemia inhibitory factor regulates microvessel density by modulating oxygen-dependent VEGF expression in mice. J Clin Invest 118: 2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe T, Raff MC (1988) Retinal astrocytes are immigrants from the optic nerve. Nature 332: 834–837. [DOI] [PubMed] [Google Scholar]
- 13. Petros TJ, Williams SE, Mason CA (2006) Temporal regulation of EphA4 in astroglia during murine retinal and optic nerve development. Mol Cell Neurosci 32: 49–66. [DOI] [PubMed] [Google Scholar]
- 14. Chan-Ling T, Chu Y, Baxter L, Weible Ii M, Hughes S (2009) In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: differentiation, proliferation, and apoptosis. Glia 57: 39–53. [DOI] [PubMed] [Google Scholar]
- 15. Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439. [DOI] [PubMed] [Google Scholar]
- 16. Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, et al. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439–442. [DOI] [PubMed] [Google Scholar]
- 17. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, et al. (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, et al. (2010) Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One 5: e11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dorrell MI, Aguilar E, Friedlander M (2002) Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci 43: 3500–3510. [PubMed] [Google Scholar]
- 20. Nakamura-Ishizu A, Kurihara T, Okuno Y, Ozawa Y, Kishi K, et al. (2012) The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. Dev Biol 363: 106–114. [DOI] [PubMed] [Google Scholar]
- 21. Ryan HE, Lo J, Johnson RS (1998) HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Criscimanna A, Duan D, Rhodes JA, Fendrich V, Wickline ED, et al. (2013) PanIN-specific regulation of Wnt signaling by HIF2. Cancer Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, et al. (2001) hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31: 85–94. [DOI] [PubMed] [Google Scholar]
- 24. Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, et al. (2009) Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci 29: 1874–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, et al. (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, et al. (2001) Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med 193: 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takeda K, Cowan A, Fong GH (2007) Essential role for prolyl hydroxylase domain protein 2 in oxygen homeostasis of the adult vascular system. Circulation 116: 774–781. [DOI] [PubMed] [Google Scholar]
- 28. Seibler J, Zevnik B, Kuter-Luks B, Andreas S, Kern H, et al. (2003) Rapid generation of inducible mouse mutants. Nucleic Acids Res 31: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, et al. (2005) Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Mol Cell Biol 25: 3163–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ho VC, Duan LJ, Cronin C, Liang BT, Fong GH (2012) Elevated vascular endothelial growth factor receptor-2 abundance contributes to increased angiogenesis in vascular endothelial growth factor receptor-1-deficient mice. Circulation 126: 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan LJ, Takeda K, Fong GH (2011) Prolyl hydroxylase domain protein 2 (PHD2) mediates oxygen-induced retinopathy in neonatal mice. Am J Pathol 178: 1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pawlinski R, Wang JG, Owens AP 3rd, Williams J, Antoniak S, et al. (2010) Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood 116: 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, et al. (2002) Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol 22: 5100–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. (1998) Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485–490. [DOI] [PubMed] [Google Scholar]
- 35. Duan LJ, Zhang-Benoit Y, Fong GH (2005) Endothelium-intrinsic requirement for Hif-2alpha during vascular development. Circulation 111: 2227–2232. [DOI] [PubMed] [Google Scholar]
- 36. Peng J, Zhang L, Drysdale L, Fong GH (2000) The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci U S A 97: 8386–8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang N, Wang L, Esko J, Giordano FJ, Huang Y, et al. (2004) Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6: 485–495. [DOI] [PubMed] [Google Scholar]
- 38. Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, et al. (2012) Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. J Clin Invest 122: 1427–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rey S, Lee K, Wang CJ, Gupta K, Chen S, et al. (2009) Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia. Proc Natl Acad Sci U S A 106: 20399–20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dioum EM, Clarke SL, Ding K, Repa JJ, Garcia JA (2008) HIF-2alpha-haploinsufficient mice have blunted retinal neovascularization due to impaired expression of a proangiogenic gene battery. Invest Ophthalmol Vis Sci 49: 2714–2720. [DOI] [PubMed] [Google Scholar]
- 41. Morita M, Ohneda O, Yamashita T, Takahashi S, Suzuki N, et al. (2003) HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J 22: 1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mowat FM, Luhmann UF, Smith AJ, Lange C, Duran Y, et al. (2010) HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PLoS One 5: e11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, et al. (1999) Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci 40: 182–189. [PubMed] [Google Scholar]
- 44. Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, et al. (2010) Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 58: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caprara C, Thiersch M, Lange C, Joly S, Samardzija M, et al. (2011) HIF1A is essential for the development of the intermediate plexus of the retinal vasculature. Invest Ophthalmol Vis Sci 52: 2109–2117. [DOI] [PubMed] [Google Scholar]
- 46. Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, et al. (2003) Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 35: 331–340. [DOI] [PubMed] [Google Scholar]
- 47. Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, et al. (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54. [DOI] [PubMed] [Google Scholar]
- 48. Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, et al. (2010) Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116: 3039–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, et al. (2006) Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol 26: 8336–8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, et al. (2008) Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood 111: 3229–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disruption of Hif-1α and Hif-2α . Pups were obtained by crossing Hif-1αf/f/Rosa26CreERT2 males with Hif-1αf/f females, or Hif-2αf/f/Rosa26CreERT2 males with Hif-2αf/f females. At P1–P3, pups were treated with tamoxifen by daily oral gavage. At P5, pups were euthanized, and retinas were dissected. Deletion of floxed Hif-1α (A) and Hif-2α (B) in retinal tissues was assessed by PCR of retinal DNA extracts. Floxed Hif-1α, 260 bp, deleted allele, 270 bp; floxed Hif-2α allele, 877 bp, deleted allele, 260 bp. HIF-1α and HIF-2α protein levels were determined by anti-HIF-1α (C) or anti-HIF-2α (D) Western blotting of retinal nuclear protein extracts.
(TIF)
Induction of more severe vascular defects in Hif-2αf/f/Rosa26CreERT2 mice by tamoxifen treatment at earlier time points. A and B. Hif-2αf/f and Hif-2αf/f/Rosa26CreERT2 neonatal mice were treated with tamoxifen at P0 through P2. C and D. Pregnant Hif-2αf/f females mated with Hif-2αf/f/Rosa26CreERT2 males were treated with a single dose of tamoxifen at 17.5 d.p.c.. Following birth, neonatal mice were treated with two more doses at P1 and P2. All retinas were stained with IB4 -Alexa 594 at P8. n = 3. Scales bar represents 50 µm.
(TIF)
GFAPCre activity in retinal astrocytes. GFAPCre mice were crossed with transgenic mice carrying a CAG promoter- loxP-Stop-loxP-tdTomato transgene targeted into the ubiquitously expressed Rosa26 locus. A to D. Co-localization of tdTomato expression with GFAP+ astrocytes. Retinas were dissected from neonatal mice at P5, and cryosections were cut at 6 µm. Sections were stained with rabbit anti-GFAP and anti-Rabbit IgG-Alexa 488, and analyzed by confocal imaging for tdTomato expression (A) or GFAP+ cells (B). Merged images are shown in C and D at different magnifications. tdTomato expression and GFAP+ cells colocalized at the inner surface of retinal tissues. E to H. Confocal images of tdTomato expression and anti-NF (neurofilament) immunofluorescence staining (green). Cryosections were prepared as in A to D, but stained with mouse anti-NF followed by goat anti-mouse IgG-DyLight 488. It is evident that tdTomato expression does not colocalize with NF+ signals. Scales bars, A–C and E to G, 100 µm; D and H, 50 µm.
(TIF)
GFAPCre activity in astrocyte progenitors. GFAPCre and Cre-inducible tdTomato transgenic mice were crossed, and pups carrying both transgenes were subject to anti-Pax2 or anti-GFAP IF staining at P0 and P3, and visualized by confocal imaging. The vast majority of tdTomato positive cells were also Pax2+ and GFAP+, demonstrating early onset of Cre activity in retinal astrocyte progenitors. n = 3. All images are at the same magnification. Scale bar represents 50 µm.
(TIF)
Hyaloid vessels in Hif-2α f/f/GFAPCre mice. Retinas were dissected at P8, care being taken to preserve hyaloid vessels. A and B. IB4-Alexa 594-stained retinas. C and D. Higher magnifications of the top right quarter from A and B, respectively. In C, white arrows point to main arterioles; green arrows indicate large branches between arterioles. Since every main arteriole has just one accompanying venules in normal retinas, additional large branches may be hyaloid vessels. Hyaloid vessels are more prominently present in Hif-2α f/f/GFAPCre mice, presumably to compensate for the loss of retinal blood vessels. n = 3.
(TIF)
Apparently normal astrocyte development in Hif-1αf/f/GFAPCre mice. At P8, retinas were dissected from Hif-1αf/f (A) and Hif-1αf/f/GFAPCre (B) mice, fixed, and stained by rabbit anti-GFAP followed by goat anti-rabbit IgG-Alexa 488. Data shown are representative confocal images from 4 mice in each genotype. Scales bars are 100 µm. Images are representative of data from 3 mice per group.
(TIF)
Astrocyte progenitors and immature astrocytes in P3 retinas. A to C are confocal images of wild-type (Hif-2αf/f) retinas stained with indicated antibodies at P3. Boxed areas are expanded and shown in D to F. Images in D to F were brightened to show more details at the periphery. These data confirm that by P3, astrocyte progenitors and immature astrocytes are already present throughout the retina. Images are representative of at least 3 mice each. Scales bars are 500 µm for A–C, and 100 µm for D to F.
(TIF)
Breeding strategy and strain background of relevant mouse lines.
(DOCX)