Abstract
A relationship between human papillomavirus (HPV) infection and papillary squamous cell carcinoma (PSCC) has been suggested. However, to date, no studies have thoroughly and directly evaluated for transcriptional activity of the virus or the clinicopathologic significance of HPV-positive PSCC. Forty-eight cases of PSCC were retrieved from our surgical pathology database and were reviewed by 4 study pathologists, with tumors defined as SCC with a significant component of papillary growth in the tumor. Immunohistochemical analysis for p16 and p53 was performed. Overexpression of p16 was used as a surrogate marker of transcriptionally active HPV. Transcriptional activity was also directly evaluated using RNA in situ hybridization to detect high-risk HPV E6/E7 mRNA. Clinical follow-up data were obtained by chart review. Seven cases were located in the oral cavity, 19 in the oropharynx, and 22 in the larynx. Two morphologic types of PSCC were identified: keratinizing type, in which the epithelial cells showed a maturation trend with minimal surface parakeratin, and nonkeratinizing type, in which the papillae were completely covered by immature basaloid cells. Transcriptionally active HPV was present in 23 of 43 (53.4%) tumors. The majority of tumors harboring transcriptionally active HPV arose in the oropharynx, showed nonkeratinizing morphology, were p16 positive, and p53 negative. Transcriptionally active HPV was also present in many laryngeal and oral cavity PSCCs. Overall survival, disease-specific survival, and disease-free survival were favorable and did not significantly differ by anatomic subsite. However, HPV-related tumors showed a trend toward better survival.
Keywords: papillary squamous cell carcinoma, head and neck, human papillomavirus, p16, RNA in situ hybridization
Papillary squamous cell carcinoma (PSCC) of the head and neck is a poorly recognized variant of SCC, which is believed to have a favorable prognosis.1-4 It is often confused with verrucous carcinoma and squamous cell carcinoma (SCC) with verrucous features. These 2 entities have broad-based exophytic projections and are characterized by excessive keratinization.5 Verrucous carcinoma does not exhibit cellular atypia, whereas SCC with verrucous features does. PSCC is characterized by a significant component of the tumor showing papillary growth with thin fibrovascular cores covered by immature basaloid cells or dysplastic squamous cells with minimal or no keratinization.1 The majority of HPV-related SCCs of the oropharynx exhibit a nonkeratinizing (NK) morphology.4,6,7 The neoplastic cells have an immature basaloid appearance and are mitotically active. Human papillomavirus (HPV) has also been identified in several head and neck SCC variants, including basaloid SCC, undifferentiated carcinoma, adenosquamous carcinoma, and, more recently, PSCC, particularly when they arise in the oropharynx but not exclusively.1,4,6 However, to date, no studies have thoroughly and directly evaluated for transcriptional activity of the HPV or for the clinicopathologic significance of its presence in PSCC.
The purpose of this study was to evaluate the prevalence of high-risk HPV in PSCC and to study the clinicopathologic features and molecular profiles of these tumors. The morphologic features of HPV-positive and HPV-negative tumors were compared with special consideration of the keratinization trends. p16 overexpression was used as a surrogate marker for transcriptionally active high-risk HPV. In addition, transcriptional activation of E6 and E7 viral oncogenes was directly assessed by in situ hybridization (ISH) for E6 and E7 mRNA. It has been shown that the presence of p53 mutation correlates with poorer survival in head and neck SCC. Several studies have shown a correlation between p53 mutation and lower response rates to chemotherapy and shorter overall survival times.8 p53 overexpression was evaluated by immunohistochemical (IHC) analysis as a surrogate marker of p53 mutation status. The clinical features and outcomes of patients with HPV-positive and HPV-negative tumors were compared.
MATERIALS AND METHODS
Case Selection and Review
After approval by the Human Research Protection Office, the surgical pathology database of the Washington University Department of Pathology and Immunology was searched for all cases diagnosed previously as “papillary squamous cell carcinoma” with no other sub-specifications as to site or terminology and all cases from the head and neck region were identified. All cases were reviewed by 4 study pathologists (M.M., S.K.E., J.S.L., R.D.C.), and tumors were defined as PSCC if they had a significant portion of papillary growth. Papillary growth consisted of full thickness dysplastic squamous cells lining fibrovascular cores. Although all cases had overtly invasive SCC components, in the papillary areas the neoplastic cells were growing within the surface epithelium with an intact basement membrane. Forty-eight cases were identified over a period of 19 years (1992 to 2011). The cases were further classified, before HPV testing, into the following 2 categories on the basis of histologic features: NK SCC and keratinizing (K) SCC. NK SCC was defined as having ovoid to spindled cells with hyperchromatic nuclei that lack prominent nucleoli and have indistinct cell borders (Figs. 1A, B). Brisk mitotic activity was often present but was not considered a requisite feature. K SCC was defined as the presence of minimal keratinization or any amount of mature squamous cells (Figs. 1C, D). This almost always was most prominent at the upper layers or the surface of the epithelium. Discrepant cases were resolved by consensus review. Clinical follow-up data were obtained by chart review from the Departments of Radiation Oncology and Otolaryngology Head and Neck Surgery at Washington University. The Social Security Death Index was searched for those patients who had been lost to follow-up, to determine the date of death, if any.
FIGURE 1.
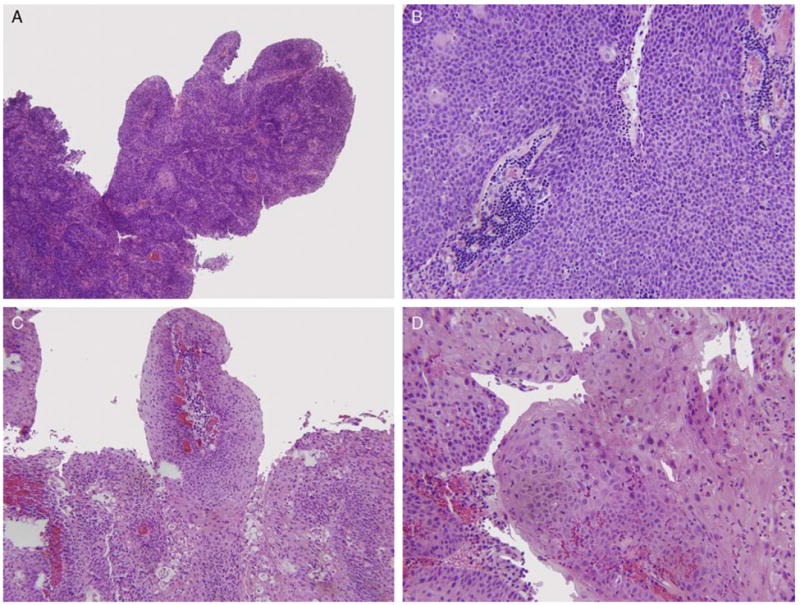
PSCC, NK type, at (A) low power and (B) high power. PSCC, K type, at (C) low power and (D) high power. All images are hematoxylin and eosin stained.
Immunohistochemistry
IHC analysis was performed using a Ventana Benchmark automated stainer using p16 (Roche MTM Laboratories; monoclonal; 1:1 dilution) and p53 antibodies (Ventana Medical Systems Inc.; monoclonal; 1:100 dilution). All cases were reviewed by 2 (M.M., S.K.E.) study pathologists. Tumors were classified as p16 positive when >50% of cells showed nuclear and cytoplasmic staining (Fig. 2A). p53 immunostaining was also classified as positive when >25% of cells showed nuclear staining. Discrepant results were resolved by consensus review.
FIGURE 2.
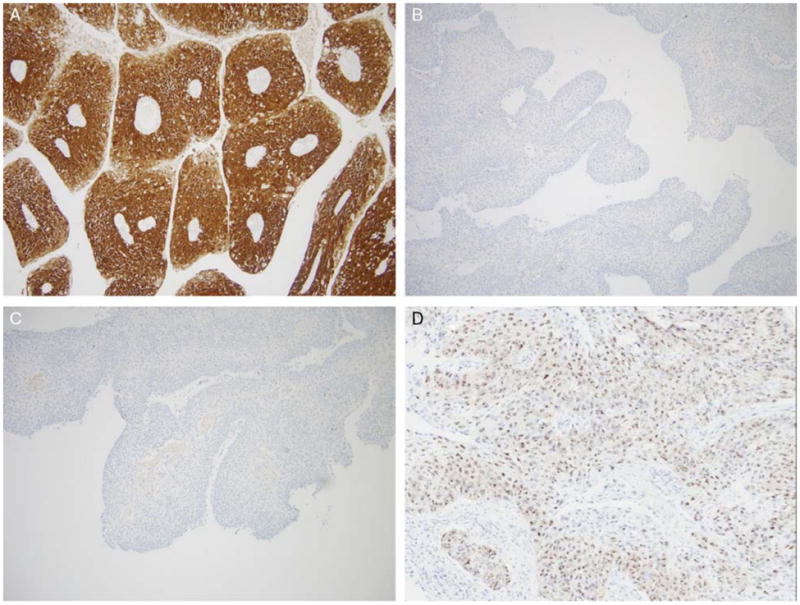
A case with (A) extensive, strong p16 positivity by IHC and (B) p53 negativity by IHC. C, A case with negative p16 IHC; and (D) RNA ISH for E6/E7 mRNA in the same case showing punctate, granular positivity.
E6 and E7 mRNA ISH
RNA ISH for E6 and E7 mRNA was performed using the RNAscope assay according to manufacturer’s instructions (Advanced Cell Diagnostics, Hayward, CA). Briefly, 4-μm-thick unstained tissue sections were pre-treated with heat and protease. Target probes to DapB (negative control), UbC (positive control), HPV-16, and high-risk HPVs as a cocktail (including types 18, 31, 33, 35, 52, and 58) were separately hybridized to adjacent tissue sections. Hybridization signals were amplified by sequential hybridization with preamplifier, amplifier, and label probes, then visualized by chromogenic staining with 3,3′-diaminobenzidine.9 Stains were reviewed by 4 (M.M., S.K.E., J.S.L., R.D.C.) study pathologists at ×20 magnification. Staining above any staining that was present on the DapB-negative control was considered positive. Cases with high background staining on the DapB-negative control or with lack of staining on the UbC-positive control were deemed technical failures.
Statistical Analysis
Categorical data were presented as frequency and percentage, whereas continuous variables were described with mean and SD. The Pearson χ2 test or the Fisher exact test as proper was used to evaluate the association between any 2 categorical data. Overall survival was defined as the time from date of commencement of the treatment (either surgical resection or beginning of radiation therapy) to the last follow-up date or death, whichever came first, whereas disease-specific survival was calculated up to the date of death with known recurrent disease. Disease-free survival was defined as the time from commencement of the treatment to the date of death due to any cause other than the disease or to the date of first disease recurrence. Empirical survival probability was estimated by the Kaplan-Meier product limit method, whereas survival differences between groups of an individual risk factor were examined by the log rank test. All tests were 2-sided with P-values ≤0.05 considered statistically significant. All the major statistical analyses were performed in R 2.14.1 (http://www.cran.R-project.org).
RESULTS
Forty-eight cases of PSCC were identified. Demographic information is presented in Table 1. There were 35 men and 13 women (ratio of 2.7: 1). The age range was from 30 to 94 years with a mean and median of 62.5 and 60, respectively. The majority of the tumors were located in the larynx (n = 22), followed by the oropharynx (n = 19) and the oral cavity (n = 7). The oral cavity tumors were diagnosed at an earlier TNM stage compared with the laryngeal tumors (P = 0.003); however, there was no significant difference in the TNM stage between the oral cavity and oropharyngeal tumors (P = 0.12) or oropharyngeal and laryngeal tumors (P = 0.11). Patients with laryngeal cancer were younger (mean age: 58.9) than their oral cavity (mean age: 67.8) and oropharyngeal (mean age: 61.5) counterparts. However, none of the age differences was statistically significant. There was no significant difference in treatment modality. Two morphologic types of PSCC were identified: the K type in which the epithelial cells showed a maturation trend with minimal surface parakeratin (Figs. 1C, D), and the NK type in which the papillae were completely covered by immature basaloid cells (Figs. 1A, B). A greater percentage of oropharyngeal PSCC cases had NK morphology compared with either oral cavity or laryngeal tumors. In the oral cavity, 1 of 7 (14.3%) was NK, whereas in the oropharynx 6 of 19 (31.5%) were NK. In the larynx, only 2 of 22 cases (9.0%) were NK (Table 2). These rates were statistically significantly different for the oropharynx versus other sites.
TABLE 1.
Distribution of 48 Cases of PSCC by Anatomic Subsite, Sex, Age, T Stage, N Stage, and Overall Stage
| Characteristics | All Sites, 48 (100) n (%) |
Oral Cavity, 7 (14.5) n (%) |
Oropharynx, 19 (39.5) n (%) |
Larynx, 22 (46.0) n (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 35 (72.9) | 3 (42.9) | 16 (84.2) | 16 (72.7) |
| Female | 13 (27.1) | 4 (57.1) | 3 (15.8) | 6 (27.3) |
| Age (y) | ||||
| Mean/median/range | 62.5/60/30-94 | 67.8/70.5/47-81 | 61.5/59/49-94 | 58.9/58.5/30-84 |
| T stage | ||||
| T1 | 11 (26.1) | 4 (80.0) | 6 (33.3) | 1 (5.2) |
| T2 | 14 (33.3) | 1 (20.0) | 6 (33.3) | 7 (36.8) |
| T3 | 11 (26.1) | 0 | 3 (16.7) | 8 (42.1) |
| T4 | 6 (14.3) | 0 | 3 (16.7) | 3 (15.8) |
| N stage | ||||
| N0 | 27 (64.2) | 5 (100) | 7 (38.8) | 15 (78.9) |
| N1 | 3 (7.1) | 0 | 1 (5.6) | 2 (10.5) |
| N2 | 11 (26.1) | 0 | 9 (50.0) | 2 (10.5) |
| N3 | 1 (2.3) | 0 | 1 (5.6) | 0 |
| Overall stage | ||||
| I | 7 (16.6) | 4 (80.0) | 2 (11.2) | 1 (5.2) |
| II | 12 (28.5) | 1 (20.0) | 4 (22.2) | 7 (36.8) |
| III | 8 (19.1) | 0 | 1 (5.6) | 7 (36.8) |
| IV | 15 (35.7) | 0 | 11 (61.1) | 4 (21.1) |
n indicates number of cases; data on TNM staging were not available for 6 cases.
TABLE 2.
Tumor Site, p16, p53, and HPV Status by RNA ISH
| Characteristics | Total | Oral Cavity | Oropharynx | Larynx |
|---|---|---|---|---|
| Morphology | ||||
| K | 39 | 6 (85.7); P = 0.012** | 6 (31.6) | 20 (81.0); P = 0.0002 |
| NK | 9 | 1 (14.3) | 13 (68.4) | 2 (9.0) |
| *p16 | ||||
| Positive | 17 | 1 (14.3); P = 0.012 | 13 (68.4) | 3 (13.6); P = 0.005 |
| Negative | 31 | 6 (85.7) | 6 (31.6) | 19 (86.4) |
| p53 | ||||
| Positive | 24 | 5 (71.4); P = 0.028 | 4 (21.1) | 15 (68.2); P = 0.004 |
| Negative | 24 | 2 (21.6) | 15 (78.9) | 7 (31.8) |
| HPV RNA ISH | ||||
| Positive | 23 | 4 (66.6); P = 0.60 | 14 (77.7) | 5 (26.3); P = 0.001 |
| Negative | 20 | 2 (33.3) | 4 (22.2) | 14 (73.6) |
p16+, >50% of tumor cells with nuclear and cytoplasmic staining; p16−, -no tumor cell staining or <50% of tumor cells with staining.
P-values are by the Fisher exact test and are for comparison of oropharynx versus oral cavity and oropharynx versus larynx, respectively.
P-values in bold are statistically significant.
Similarly, more oropharyngeal PSCCs were p16 positive (but fewer were p53 positive) than oral cavity or laryngeal tumors. One of 7 (14.3%) oral cavity tumors was positive for p16, and 5 of 7 (71.4%) were positive for p53. In the oropharynx, 13 of 19 (68.4%) cases were positive for p16, and 4 of 19 (21.1%) were positive for p53. Three of 22 (13.6%) laryngeal cases were positive for p16, and 15 of 22 (68.2%) were positive for p53. The prevalence of p16 overexpression in oropharyngeal tumors was significantly higher than that of the oral cavity and larynx (P = 0.012 and 0.005, respectively). Conversely, p53 expression in the oropharyngeal cases was significantly lower compared with that in the oral cavity and larynx (P = 0.028 and 0.004, respectively) (Table 2). Patients with p16-positive tumors were younger in general compared with those having p16-negative tumors, but the difference was not statistically significant (P = 0.08) (Table 3). There were no other differences among the other clinical characteristics in terms of either p16 or p53 status.
TABLE 3.
Age, Morphology, p53, and RNA ISH Status by p16 Expression
| Characteristics | n | p16+ (%)* | p16− (%) | Overall P |
|---|---|---|---|---|
| Total | 48 | 17 (34.0) | 31 (66.0) | |
| Mean age/SD (y) | 57/8.3 | 63/13.1 | 0.08 | |
| Morphology | 0.0001 | |||
| K | 32 | 4 (23.5) | 28 (90.3) | |
| NK | 16 | 13 (76.5) | 3 (9.7) | |
| p53 | 0.0002 | |||
| Positive | 24 | 2 (11.8) | 22 (71.0) | |
| Negative | 24 | 15 (88.2) | 9 (29.0) | |
| HPV RNA ISH | 0.0001 | |||
| Positive | 23 | 16 (100) | 7 (25.9) | |
| Negative | 20 | 0 (0)† | 20 (74.1)‡ |
p16+, >50% of tumor cells with nuclear and cytoplasmic staining; p16−, no tumor cell staining or <50% of tumor cells with staining.
One p16+ case had technical failure on RNA ISH.
Four p16− cases had technical failure on RNA ISH.
n indicates number of cases.
P-values in bold are statistically significant.
HPV was detected by RNA ISH in the majority of oropharyngeal and oral cavity PSCCs but also in a minority of laryngeal PSCCs. HPV E6/E7 mRNA was identified by ISH in 14 of 18 (77.7%) oropharyngeal PSCC cases, whereas in the oral cavity, 4 of 6 (66.6%) were positive. These proportions were not significantly different (P = 0.60). In contrast, only 5 of 19 (26.3%) cases of laryngeal PSCC were positive for E6/E7 mRNA, which was significantly less than that in the oropharynx (P = 0.001) (Table 3). There were 5 RNA ISH technical failures (1 oral, 1 oropharyngeal, and 3 laryngeal cases). There was 94.4% concordance between HPV status by RNA ISH and p16 IHC in the oropharynx and 84.2% in the larynx. However, this concordance declined to only 50.0% in the oral cavity. There were no p16-positive, HPV RNA–negative cases, but 7 cases were p16 negative and HPV RNA ISH positive. Three of these were from the oral cavity, 1 from the oropharynx, and 3 from the larynx.
The patients were followed up for a mean duration of 32.5 months. Overall, 16 patients had lymph node metastasis at the time of diagnosis (33.3%), including 11 of 18 (61.1%) from the oropharynx and 5 of 19 (26.3%) from the larynx. None of the oral cavity tumors had evidence of lymph node metastasis at the time of diagnosis. Five additional patients developed lymph node metastasis later in the course of their disease, 2 from the oral cavity and 3 from the larynx. Overall, the prognosis of PSCC was quite favorable. Tumor recurred locally in only 8 of 42 (19.0%) patients, and distant metastasis occurred in only 3 patients (7.1%). Two of these patients had primary tumors in the larynx and 1 in the oropharynx. None of the oral cavity tumors had distant metastasis. Overall, 22 patients (45.8%) died during the follow-up period, but only 9 (18.7%) died directly from their cancers.
On univariate analysis, overall, disease-specific, and disease-free survival did not differ significantly by anatomic subsite, histology, stage, sex, age, or treatment modality. However, although not statistically significant, p16-positive tumors did show trends toward improved overall, disease-specific, and disease-free survival (Table 4; Fig. 3).
TABLE 4.
Patient Survival by p16 IHC Status
| 5 y Overall Survival (%) | 5 y Disease-free Survival (%) | 5 y Disease-specific Survival (%) | |
|---|---|---|---|
| p16+* | 52 | 58 | 80 |
| p16− | 54 | 48 | 73 |
p16+, >50% of tumor cells with nuclear and cytoplasmic staining; p16−, no tumor cell staining or <50% of tumor cells with staining.
FIGURE 3.
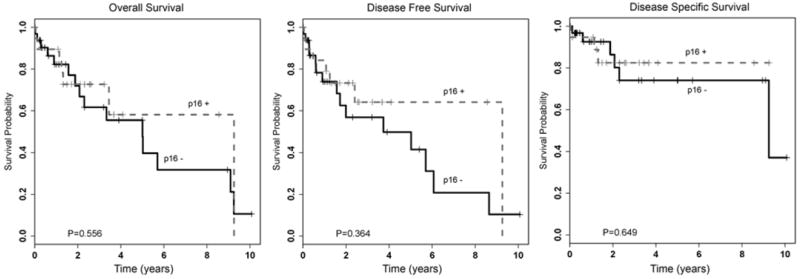
Kaplan-Meier survival curves for p16-positive and p16-negative patient cohorts. (p16 positive, >50% of tumor cells with nuclear and cytoplasmic staining; p16 negative, no tumor cell staining or staining in <50% of tumor cells).
DISCUSSION
Several series of head and neck PSCC cases have been published. Ishiyama et al10 studied 52 cases in regard to their biological behavior, but the relationship with HPV was not reported. Thompson et al11 reviewed the histomorphology and outcomes of 104 PSCC cases and studied the HPV status by DNA-based ISH in 41 cases. In their study, only 1 of 41 cases tested was reactive for HPV types 6/11, and none demonstrated reactivity with high-risk HPV. Suarez et al12 published 38 cases of this tumor with clinical follow-up information. In their series, HPV detection was carried out in 14 cases by DNA-based ISH and polymerase chain reaction (PCR). HPV was demonstrated in 4 of 14 (29%) tumors by ISH and in 5 of 14 (43%) tumors by PCR. Two additional cases were positive for HPV 6/11 by ISH and PCR, and 3 of 4 tumors showed HPV-16/18 by PCR in which ISH results were negative. Jo et al13 studied 31 PSCCs of the upper aerodigestive tract by p16 IHC and DNA-based ISH for high-risk HPV status and reported clinical follow-up information. They found 23 of 31 (74.1%) cases to be p16 positive, and all 15 of the p16-positive lesions that were tested were positive for high-risk HPV by DNA-based ISH (68.1%). Russell et al14 recently studied 52 cases of PSCC for demographics and survival rates and showed favorable prognosis. But no HPV studies were performed.
In the current study, we found almost half (23 of 48; 47.9%) of the PSCC cases to harbor transcriptionally active high-risk HPV including the majority of oropharyngeal and oral cavity cases and a minority of laryngeal ones. Most (17 of 23; 73.9%) of the HPV RNA–positive cases were p16 positive as well. There is a fairly substantial amount of literature on the correlation between HPV DNA detection, p16 IHC, and HPV RNA detection. p16-positive, HPV-negative head and neck PSCCs have been previously described.13 In the study by Jo and colleagues, 32% of p16-positive tumors were negative on HPV DNA ISH. This has also been described in studies in the literature on conventional SCC with approximately 5% to 15% of oropharyngeal and significantly more nonoropharyngeal SCC cases being p16 positive without detectable HPV. In a recently published article on all subsite head and neck SCCs, Bishop et al15 showed that p16 expression was strongly associated with the presence of HPV E6/E7 mRNA by RNA ISH. Forty six of 49 HPV-positive tumors were extensively p16 positive, whereas only 22 of 233 HPV-negative tumors were p16 positive (94% vs. 9%; P < 0.0001). There were 3 HPV RNA ISH–positive, p16-negative tumors, representing 6% of all HPV RNA–positive cases. Ukpo et al16 performed RNA ISH for high-risk HPV E6/E7 mRNA and p16 IHC on cases of oropharyngeal SCC and showed that HPV RNA ISH is highly concordant with p16 IHC. They found a total of 4 cases in which p16 was negative and HPV RNA ISH was positive, representing 2.6% of the 152 RNA ISH–positive cases. Rather than representing technical problems with the assays, these cases appear to reflect rare examples of HPV-positive SCCs truly lacking p16 expression. In our study on PSCC, all p16-positive tumors were also HPV RNA ISH positive. In contrast, we found 7 of 23 (30.4%) of our HPV RNA ISH–positive tumors to be p16 negative (Figs. 2C, D). It is possible that in a minority of cases, particularly in the oral cavity, the transcriptional activation of HPV is not associated with overexpression of p16 whether as a fundamental biological mechanism of the HPV in the tumor cells or as acquired inactivation of p16 expression after HPV activation. This argues that p16 IHC, which is widely regarded as highly sensitive for the presence of transcriptionally active high-risk HPV, may not be a good surrogate marker or screening test for HPV in PSCC, at least for nonoropharyngeal cases. However, the number of cases is too small to derive any definite conclusions.
In the head and neck region, HPV-related SCCs present most commonly in the oropharynx, show distinct NK morphology, and are associated with favorable patient outcomes.1,11,12,17-19 More recently, HPV has been etiologically linked to several SCC variants in the oropharynx and occasionally in nonoropharyngeal head and neck anatomic subsites. These variants include basaloid SCC, undifferentiated carcinoma, adenosquamous carcinoma, small cell carcinoma, and PSCC. Evidence shows that most of these variants are associated with better prognosis than their HPV-negative counterparts, although the number of cases is generally small.6,7,13,20 Although some studies have investigated the association of HPV with PSCC,4,6,8,11-13 to date none have described the histologic keratinization patterns nor have tested the tumors directly for the transcriptional activity of high-risk HPV by identifying E6/E7 mRNA in the tumor cells.
In our series we found similar demographic features for PSCC as in the previous studies.1,4,6 None of our patients had preexisting squamous papillomas or leukoplakia, these findings being in agreement with the series by Russell et al14 and Ishiyama et al.10 Our findings and literature on this issue strongly argue that SCC arises de novo in almost all cases rather than as progression to malignancy in patients with papillomatosis.
In the present study, we have demonstrated that a significant subset of PSCC cases are etiologically related to HPV. The virus in these tumors is transcriptionally active as demonstrated both indirectly by p16 overexpression and directly by positive E6/E7 mRNA ISH. HPV-related PSCC is most commonly found in the oropharynx, wherein p16 overexpression and positive RNA ISH were detected in 68.4% and 77.7% of the cases, respectively. These tumors frequently had NK morphology and were more likely p53 negative (Fig. 2B). The prevalence of HPV in oropharyngeal PSCC paralleled that observed in the typical (or “nonpapillary”) HPV-related NK SCC of the oropharynx.1,4,6,7
HPV was less prevalent in the larynx, but was present in a significant minority of cases, with p16 positivity in 13.6% and HPV RNA ISH positivity in 26.3% of the cases. Most of these tumors showed K morphology. Again, the HPV-related PSCC cases showed some similarities to conventional HPV-related SCC in prevalence and morphologic trends.11 In the oral cavity, the prevalence of transcriptionally active E6/E7-positive ISH in PSCC in our series (66.6%) far exceeded the reported prevalence of 0% to 10% for conventional SCC at this subsite.13,21,22 Although the number of the oral cavity and laryngeal cases in this study is rather limited to reach any definitive conclusions, it is very suggestive that PSCC of the oral cavity, and to a lesser extent the larynx, is more likely to be associated with transcriptionally active HPV than conventional SCC. This is a novel and unusual finding for PSCC because very few nonoropharyngeal SCC (conventional or even the histologic variants such as basaloid SCC and undifferentiated, verrucous, and adenosquamous carcinomas) harbor transcriptionally active HPV when arising in the larynx or oral cavity.
Interestingly, some of the HPV-related tumors showed a morphologic trend toward keratinization as reported in the rare HPV-related conventional SCCs described in the oral cavity.11,22 We observed an inverse relationship between HPV-positive tumors and p53 expression, as has been well demonstrated in the overall majority of HPV-related SCC of the head and neck. Jo et al13 described 11 of their 14 PSCCs to be positive for p53 protein and that the p53 status was significantly related to poor survival (P = 0.048). In addition, all of their HPV-negative PSCCs had 4+ (extensive) staining for the p53 protein. This again is similar to our findings.
We have shown that oropharyngeal PSCCs differ significantly from their counterparts in the oral cavity and the larynx. They tend to have an NK morphology and are more frequently associated with transcriptionally active HPV. We have further demonstrated that PSCC of the upper aerodigestive tract has 2 distinct morphologic types, K and NK. The majority of the NK PSCCs are located in the oropharynx, are more associated with high-risk HPV, and occur in a younger population of patients compared with the oral cavity and laryngeal lesions. In addition, a number of oral cavity and laryngeal PSCCs are also related to transcriptionally active high-risk HPV, most of which lack NK morphology.
The question remains as to whether, and what, HPV testing should be performed in PSCC in routine clinical practice. We test oropharyngeal SCC because there is overwhelming evidence for the clinical and therapeutic significance of transcriptionally active HPV.16,22-24 Our current practice, just as with many others across the head and neck community, is to routinely test all oropharyngeal SCCs, including all histologic variants, for p16 (as a surrogate marker of HPV and as a proven prognostic marker). It is acknowledged that many feel compelled to perform some type of HPV-specific testing in oropharyngeal SCC as well.25,26
We do not test oral cavity, laryngeal, or hypopharyngeal SCC cases for HPV or p16 in routine practice because no definitive evidence exists to support its clinical significance at these anatomic subsites. Even though there are many PSCCs in the larynx and oral cavity that harbor transcriptionally active HPV, it has not been shown that they are biologically different from HPV-negative ones or that they should be treated differently. In summary, given the current state of knowledge, we recommend HPV testing in routine practice only for oropharyngeal PSCC.
This study further supports the overall good prognosis of head and neck PSCCs previously described in the literature. However, it should be noted that most series report high rates of local recurrence and also of secondary malignancies.8,10,12,13,14 In our series, the HPV-related tumors were not associated with statistically significant improved patient outcomes, although the HPV-positive tumors did tend to have better survival compared with the HPV-negative ones (Fig. 3). The lack of significant difference in survival by HPV status could be explained by the relatively small sample size, or it could be attributable to the overall favorable prognosis of PSCC.
Acknowledgments
The authors acknowledge Jianping Li, BS and Neha Dahiya, MD, MBA of the Anatomic and Molecular Pathology Core Laboratory for their expert assistance with the p16 and p53 IHC. The authors also acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center, and NCI Cancer Center Support Grant P30 CA091842.
Source of Funding: The RNA in situ hybridization assays in the work were funded and performed by the coauthors from Advanced Cell Diagnostics Inc., Hayward, CA. Three coauthors, H.W., Y.L., X.-J.M., have stock in this company and stand to profit by any use of this testing through their company.
Footnotes
Presented at the United States and Canadian Academy of Pathology, 101st Annual Meeting, Vancouver, BC, Canada, 2012.
Conflicts of Interest
For the remaining authors none were declared.
References
- 1.Cardesa A, Zidar N, Nadal A, et al. Papillary squamous cell carcinoma. Pathology and Genetics of Head and Neck Tumours. In: Kleihues P, Sobin LH, Barnes EL, Eveson JW, Reichart P, et al., editors. World Health Organization Classification of Tumours. Lyons, France: IARC Press; 2005. p. 126. series editors. [Google Scholar]
- 2.Cobo F, Talavera P, Concha A. Review article: relationship of human papillomavirus with papillary squamous cell carcinoma of the upper aerodigestive tract: a review. Int J Surg Pathol. 2008;16:127–136. doi: 10.1177/1066896908314700. [DOI] [PubMed] [Google Scholar]
- 3.Crissman JD, Kessis T, Shah KV, et al. Squamous papillary neoplasia of the adult upper aerodigestive tract. Hum Pathol. 1988;19:1387–1396. doi: 10.1016/s0046-8177(88)80231-4. [DOI] [PubMed] [Google Scholar]
- 4.El-Mofty SK. HPV-related squamous cell carcinoma variants in the head and neck. Head Neck Pathol. 2012;6(suppl 1):S55–S62. doi: 10.1007/s12105-012-0363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlito A, Devaney KO, Rinaldo A, et al. Papillary squamous cell carcinoma versus verrucous squamous cell carcinoma of the head and neck. Ann Otol Rhinol Laryngol. 1999;108:318–322. doi: 10.1177/000348949910800318. [DOI] [PubMed] [Google Scholar]
- 6.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27:1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 7.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiyama A, Eversole LR, Ross DA, et al. Papillary squamous neoplasms of the head and neck. Laryngoscope. 1994;104:1446–1452. doi: 10.1288/00005537-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Thompson LD, Wenig BM, Heffner DK, et al. Exophytic and papillary squamous cell carcinomas of the larynx: a clinicopathologic series of 104 cases. Otolaryngol Head Neck Surg. 1999;120:718–724. doi: 10.1053/hn.1999.v120.a92773. [DOI] [PubMed] [Google Scholar]
- 12.Suarez PA, Adler-Storthz K, Luna MA, et al. Papillary squamous cell carcinomas of the upper aerodigestive tract: a clinicopathologic and molecular study. Head Neck. 2000;22:360–368. doi: 10.1002/1097-0347(200007)22:4<360::aid-hed8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Jo VY, Mills SE, Stoler MH, et al. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33:1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 14.Russell JO, Hoschar AP, Scharpf J. Papillary squamous cell carcinoma of the head and neck: a clinicopathologic series. Am J Otolaryngol. 2011;32:557–563. doi: 10.1016/j.amjoto.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JS, Jr, Ukpo OC, Ma XJ, et al. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas—a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathology. 2012;60:982–991. doi: 10.1111/j.1365-2559.2011.04169.x. [DOI] [PubMed] [Google Scholar]
- 18.Sedaghat AR, Zhang Z, Begum S, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119:1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 19.Yamakawa-Kakuta Y, Kawamata H, Doi Y, et al. Does the expression of HPV16/18 E6/E7 in head and neck squamous cell carcinomas relate to their clinicopathological characteristics? Int J Oncol. 2009;35:983–988. doi: 10.3892/ijo_00000412. [DOI] [PubMed] [Google Scholar]
- 20.Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 21.Ha PK, Pai SI, Westra WH, et al. Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res. 2002;8:1203–1209. [PubMed] [Google Scholar]
- 22.Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS. 2010;118:510–519. doi: 10.1111/j.1600-0463.2010.02624.x. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JS, Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27:6213–6221. doi: 10.1200/JCO.2009.23.1670. [DOI] [PubMed] [Google Scholar]
- 25.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 26.Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]


