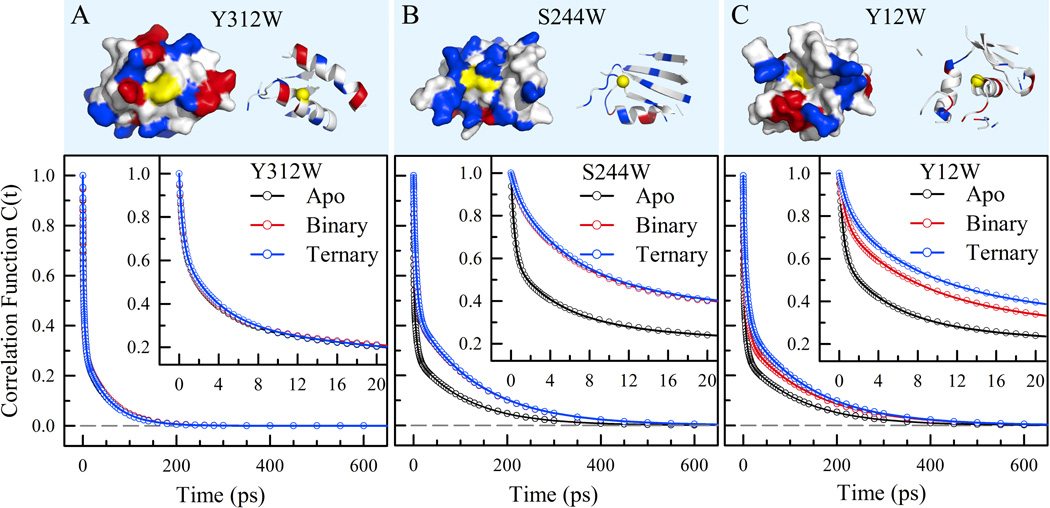
Figure 4.
Local protein properties and solvation correlation functions for the mutant Y312W (A), S244W (B) and Y12W (C) of Dpo4 in the apo, binary and ternary states. (Upper) Surface-map and ribbon representations of the local structures within 12Å from the tryptophan probe with positive (blue), negative (red) and neutral (white) residues as well as mutation sites (yellow). The yellow balls in the ribbon structures indicate the specific mutation sites. (Lower) Solvation correlation functions in three different states. The circles are the derived experimental data and the solid lines are the best exponential fit. Y312W as a control shows no changes in the three states, S244W in the binding site results in slowdown from the apo state to the complex states, and Y12W in the active site shows gradual changes from the apo, to binary and to ternary states.