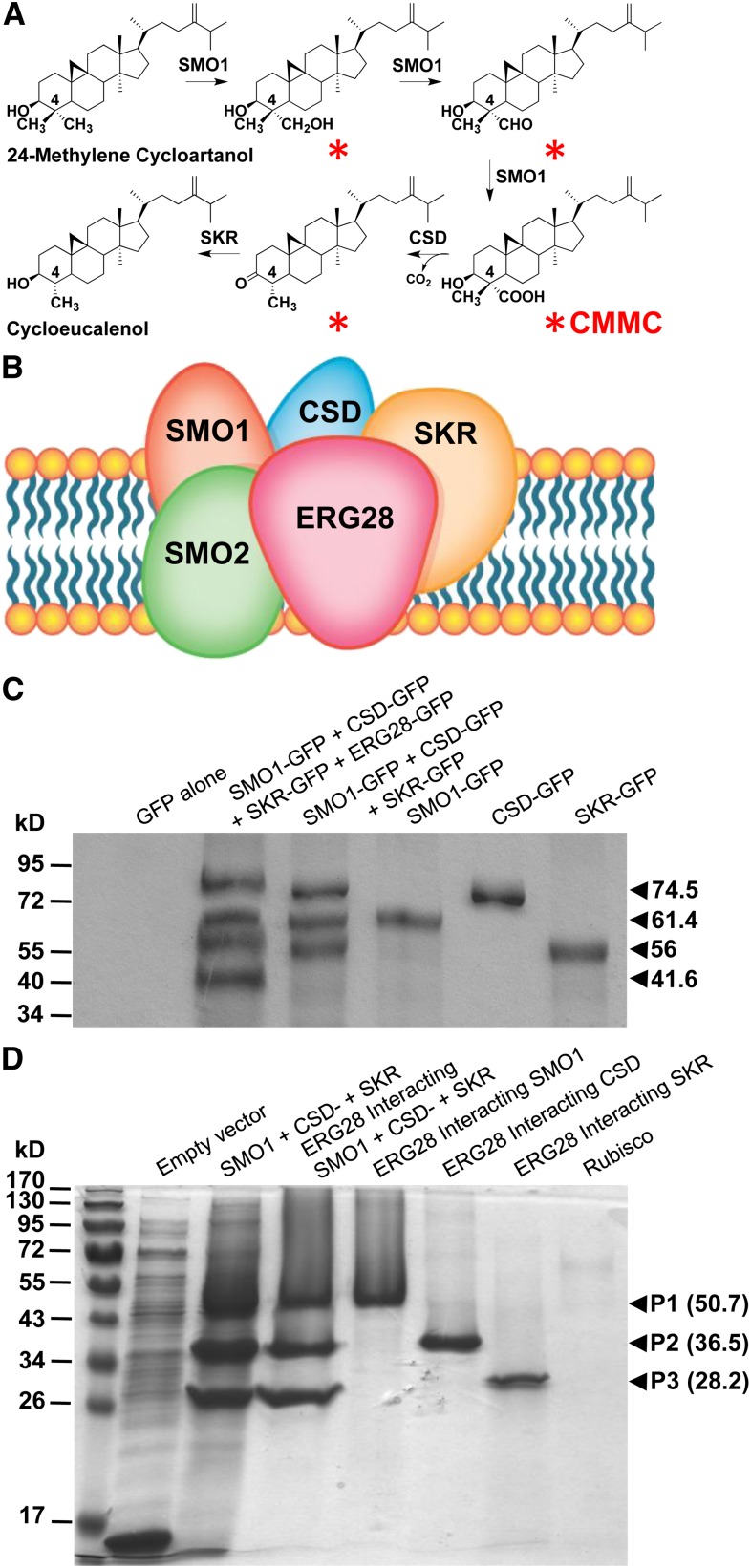
Figure 1.
Arabidopsis ERG28 Tethers the SC4DM Multienzyme Complex Catalyzing the Production of C4-Methyl SBIs.
(A) Four SBIs (red asterisks) including CMMC are sequentially formed but not released from the enzyme supercomplex during the conversion of 24-methylene cycloartanol to cycloeucalenol. The reaction is catalyzed by SC4DM component enzymes (SMO1, sterol 4α-methyl oxidase; CSD, 4α-carboxysterol-C3-dehydrogenase/C4-decarboxylase; and SKR, sterone keto-reductase) tethered by ERG28 to promote the channeling of SBIs (for additional steps catalyzed by the SC4DM multienzyme complex, see Supplemental Figure 1 online).
(B) Model predicting the tethering of SC4DM multienzyme complex by ERG28 based on yeast data.
(C) Coimmunoprecipitation of SC4DM-GFP component enzymes (SMO1-GFP, CSD-GFP, and SKR-GFP) from leaves transfected with constructs as shown above the gel lanes using anti-Arabidopsis ERG28 coupled to sepharose beads and immunoblot analysis with anti-GFP.
(D) In vitro interactions between Arabidopsis ERG28 and the SC4DM component enzymes. Biotinylated recombinant Arabidopsis ERG28 attached to streptavidin agarose was used as a bait to pull down recombinant Arabidopsis: SMO1, CSD, and SKR. Rubisco was used as a negative control bait. Soluble extracts from Escherichia coli overexpressing recombinant SMO1 (1 to 298), CSD (134 to 322), and SKR (98 to 229) as thioredoxin fusion proteins were incubated in a 1:1:1 (v:v:v) ratio or individually with purified biotinylated ERG28 or Rubisco immobilized on agarose beads before elution. SDS-PAGE analysis of soluble bacterial lysate from induced bacteria harboring an empty vector or a mixture of soluble proteins from induced bacteria expressing SMO1, CSD, and SKR and ERG28-interacting proteins.
[See online article for color version of this figure.]