Graphical abstract
Highlights
► Costs of GI parasite infection are detectable with hematological indices. ► With aggregate parasite data, costs of infection were detected in male buffalo. ► Species-specific parasite data revealed hidden costs in female buffalo. ► Hematological data revealed costs not evident with standard body condition data. ► Parasite species richness and identity are important for measuring infection costs.
Keywords: Helminth, Condition, Parasite species richness, African buffalo
Abstract
Most animals are concurrently infected with multiple parasites, and interactions among these parasites may influence both disease dynamics and host fitness. However, the sublethal costs of parasite infections are difficult to measure and the effects of concomitant infections with multiple parasite species on individual physiology and fitness are poorly described for wild hosts. To understand the direct and indirect physiological costs of co-infection, we investigated the relationships among gastrointestinal parasite richness, species identity, and abundance and host hematological parameters, body condition, and investment in lymphocyte defenses. Using aggregate-scale parasite data from African buffalo (Syncerus caffer), we found few direct or indirect associations between infection and hematology in male hosts, and no significant associations were observed in female hosts or with respect to body condition in either sex. These results suggest that only strong physiological effects are detectable with aggregate-scale parasite data, and that hematological variables may be more sensitive to changes in condition than standard body fat condition indices. Analyses accounting for parasite species identity in female buffalo revealed that different parasites show distinct relationships with host hematology, body condition, and immune investment. However, four of six species-specific associations were obscured when parasites were considered in combination. Overall, fitness-related physiological mediators such as hematological indices may provide assessments of direct and indirect effects of parasite infection, particularly when parasite species identity and community composition are considered.
1. Introduction
Both microparasites (e.g. bacteria, viruses) and macroparasites (e.g. helminths, arthropods) can have far-reaching effects on the fitness of their hosts, ranging from reducing body condition below levels critical for reproduction to causing castration or direct mortality (Berger et al., 1998; Stien et al., 2002; Lafferty and Kuris, 2009). The effects of parasites on host population dynamics have also been documented under a range of conditions and in an increasing number of host taxa (Tompkins and Begon, 1999; Tompkins et al., 2010). Historically, most studies describing parasite effects on individual hosts or host populations focused on single parasite species, despite the fact that an overwhelming majority of hosts are simultaneously infected with multiple parasites (Petney and Andrews, 1998). More recently, however, both the number and specific identity of co-infecting parasites have been implicated as potentially critical determinants of the relative impact of parasites on hosts (Behnke et al., 2005; Craig et al., 2008; Telfer et al., 2010). These studies highlight the need for additional work focused on co-infection as it relates to host fitness and population dynamics.
Even for single parasite infections, the fitness costs of infection can be difficult to quantify in the wild. For example, in short-term or cross-sectional wildlife studies, host survival is rarely quantified, infection duration is unknown, and reproduction is typically calculated in terms of offspring produced during a single breeding period. Accordingly, estimates of parasite effects on host fitness are often difficult to determine. Longitudinal and experimental studies can provide accurate information on the costs of parasite infection when effects of parasites on host survival and/or reproduction are monitored over time (e.g. Hudson et al., 1998; Stien et al., 2002; Telfer et al., 2008). However, the logistical difficulty and expense of these studies mean that comparatively few such studies can realistically be conducted in wild systems. When longitudinal studies or experiments are not feasible, another alternative may be for short-term studies to use physiological performance indices as a currency for inferring the downstream costs of parasitism to the host.
Physiological indices (e.g. hormones, immunity, energetics) are intrinsically connected to performance and reproductive success (Moore and Hopkins, 2009). Ecologists often measure energetic reserves by assessing stored fat, mass, or mass/size ratios as a measure of individual body condition. Alternatively, hematological profiles, including measures of the size and abundance of red blood cells and counts of white blood cell types, respond quickly to physiological perturbations (i.e. diet, stress, hydration status), and are widely used by veterinarians and physicians as indices of condition (Jain, 1993; Yochem et al., 2008). Although rarely used by ecologists, hematological measures may provide additional or potentially more sensitive information on the physiological status of the host.
Hematological profiles respond to parasite infection as a direct result of parasite-induced blood and energy losses (Colditz, 2008), up-regulation of host immunity in response to infection, and even the repair of collateral damage caused by host immune mediators (Lochmiller and Deerenberg, 2000; Colditz, 2008). For instance, some species of gastrointestinal (GI) helminths remove red blood cells from circulation by directly sucking host blood (e.g. barber pole worms (Haemonchus sp.) in ruminants) or by inflicting intestinal lesions (e.g. hookworms in humans). This form of chronic blood loss alters the host’s hematological profile by reducing red blood cell counts and volume in the short term, and by gradually impairing red blood cell regeneration over the long-term, leading to the production of small, hemoglobin-deficient red blood cells (Harmening, 1997). Non-bloodsucking helminths (e.g. Cooperia sp. and Trichostrongylus sp. in ruminants) can also alter host hematology by limiting essential nutrients (e.g. amino acids, copper, protein) or as a byproduct of the immune response to chronic infection (Feldman et al., 2000). Ultimately, parasite-induced hematological changes can increase host morbidity and/or mortality (Chambellan et al., 2005; Qiu et al., 2010; Rodrigues et al., 2010), and decrease reproductive output (Bearhop et al., 1999; Allen, 2000; Ramakrishnan, 2001; Table 1). Thus, examining hematological parameters, in addition to standard body condition indices, may provide an integrated, short-term measure of the effect of parasites on hosts.
Table 1.
Hematological parameters assessed in this study, including the typical response to parasite infection and their reported associations with reproduction and survival in birds and mammals. MCV has a more variable relationship with parasite infection, possibly because it depends on the relative magnitude of changes in RBC and HCT.
| Parameter | Definitionl | Biological significancel | Response to parasite infection | Association with reproduction | Association with survival |
|---|---|---|---|---|---|
| Red blood cells (RBC) | Number of red blood cells in a set volume of blood | Aerobic capacity, blood loss, regeneration | Decreasea,b | ||
| Hemoglobin (HG) | Amount of hemoglobin protein in a set volume of blood | Oxygen binding capacity of blood | Decreasea,b,c | Positived | Positivee,f |
| Hematocrit (HCT) | Percentage of red blood cells in a set volume of blood | Number and size of RBC, aerobic capacity | Decreasea,b,c,g,h | Positived | Positivei |
| Mean corpuscular volume (MCV) | Average size of red blood cells (HCT/RBC) | RBC age and regeneration | Decreasea or Increaseb | Positivej,k |
In this study, we examined the relationships between multiple GI parasite infections and host hematological profiles and body condition in free-ranging African buffalo (Syncerus caffer). Non-invasive GI parasite identification and quantification is typically based on the number and types of infective stages shed in host feces (Ezenwa and Jolles, 2008; Turner and Getz, 2010), but the infective stages of many GI parasites are similar in appearance and are morphologically identifiable only to order or family-level (e.g. the strongyle nematodes). Recent advances in molecular techniques allow for more detailed identification of parasite eggs and larvae (Zarlenga and Higgins, 2001; Gasser et al., 2008; Bott et al., 2009), and here we used a combination of morphology-based aggregate parasite data and molecular-based species-level data to examine the extent to which information on parasite species identity improves inferences about parasite effects on co-infected hosts. Using aggregate parasite data, we examined the direct effects of parasite richness and egg abundance on four distinct hematological parameters indicative of blood oxygen carrying capacity and red blood cell quantity and regenerative ability (see Table 1). We also tested for indirect effects of immune investment, quantified as numbers of lymphocytes, on red blood cell parameters, since immune defenses are energetically costly to develop and maintain, and may indirectly affect host physiology and performance (Moore and Hopkins, 2009). Finally, using species-specific parasite data, we examined the degree to which parasite identity influenced host costs in terms of hematological profiles and immune investment. In all cases, we compared results based on host hematological parameters to results using a more traditional index of host body condition.
Overall, we expected that: (1) aggregate parasite richness and egg abundance would be negatively associated with host physiological indices, reflected by declines in hematological indices and body condition; (2) investment in lymphocytes would be positively correlated with parasite richness and abundance, and show independent negative associations with hematological parameters and condition; and (3) parasite species identity would play a key role in the type and magnitude of observed effects, since the effects of blood-feeding and non-blood feeding parasites are likely manifest through different hematological parameters. Throughout, we expected that if parasites were exerting costs on the host, rather than vice versa, this would be apparent as consistent negative associations between hematology, body condition and parasite infection, or between immune investment and physiological indices. We also expected that parasite species-specific differences in detectable costs would lend support to the idea that observed associations reflect parasite effects on the host rather than host effects on the parasites.
2. Materials and methods
2.1. Background
African buffalo were sampled from Hluhluwe-iMfolozi Park (HIP) and Kruger National Park (KNP), South Africa. In HIP, herds were sampled over two weeks in the Masinda section of the park in October 2005 (dry season) and again in May 2006 (wet season). Buffalo were captured by HIP management by funneling animals into a corral using a helicopter. In KNP, animal capture was carried out by South Africa National Parks Veterinary Wildlife Services in June/July 2008 (wet season) and October 2008 (dry season). Buffalo herds were located by helicopter and females between the ages of 2–3 were preferentially targeted for darting due to the needs of a concurrent study. Captured buffalo were branded (HIP, KNP) and fitted with radiocollars (KNP) to prevent re-sampling. Overall, 203 males (HIP only) and 278 females (134 from HIP and 144 from KNP) were sampled.
Once animals were captured and immobilized, we collected the same suite of host trait data on all individuals at both study sites including age, sex, body condition and reproductive status (females only). Buffalo age was estimated based on body size and horn development for individuals under 2.5 years old (Sinclair, 1977). Incisor eruption patterns were used to estimate age for animals from 2.5 to 5.5 years old. For older individuals (>5.5 years), tooth wear of the first incisor was used to estimate age (Jolles et al., 2005). To assess body condition, we used an integrated body fat score based on the manual palpation and visual inspection of four areas of the body where buffalo store fat (Ezenwa et al., 2009). This body condition index has been shown to be correlated with the kidney fat index, a widely used measure of body condition in ungulates (Ezenwa et al., 2009). Finally, pregnancy status was evaluated via rectal palpation and lactation status was assessed by manual milking of all four teats.
2.2. Hematological parameters
We collected blood samples from all individuals for hematological analysis. Samples were collected via jugular venipuncture into 10 ml EDTA tubes and placed on ice immediately after collection until processing. Blood samples from HIP were shipped to Dr. Bouwer & Partners Inc. (Durban, South Africa) and analyzed for hematological parameters and white blood cell differential counts using an ADVIA 120 automated analyzer. Samples were processed within 3 days of collection. For the KNP samples, hematological parameters were measured using an ABX ABC Vet hematological analyzer and samples were processed on the day of collection. Blood smears were made within 8 h of blood collection and white blood cell differential counts were performed manually by a single observer using a compound microscope. The four red blood cell measures used and their medical and ecological importance are described in Table 1. The main effector cells of the adaptive immune system are B and T lymphocytes (Janeway, 2008). As such, the proportion of white blood cells that were lymphocytes was used to assess constitutive adaptive immune function.
2.3. GI parasite assessment
To assess GI parasite richness and egg abundance, we used fecal samples collected directly from the rectum of each immobilized animal. After collection, samples were placed on ice while in the field and at 4 °C for 0–5 days until processing. GI parasites were quantified following a modified McMaster egg counting protocol (Ezenwa, 2003). Five aggregate classes of parasites were distinguished including: Monezia sp. (Platyhelminthes: Anoplocephalidae), Trichuris sp. (Nematoda: Trichuridae), coccidia (Apicomplexa: Eimeriidae), Strongyloides sp. (Nematoda: Strongyloididea), and strongyle nematodes (Nematoda: Trichostrongylidae). We defined parasite richness as the total number of parasite types present in a sample. We used the number of strongyle nematode eggs per gram of feces (egg abundance) as an index of the energetic burden of parasite infection since this was the dominant parasite type and most of the resources taken from the host by nematodes are converted into eggs (Jennings and Calow, 1975; Combes, 2001).
For species-specific analyses, we identified strongyle nematodes to species in a subset of the individuals captured in KNP (n = 30). To do this, we isolated third-stage larvae from fecal samples using a modified Baermann technique, which included culturing up to 35 g of feces for approximately 10 days to allow eggs to hatch then collecting individual larvae. Cultures were inspected and stirred every 3 days to prevent fungal growth. After 10 days, culture jars were filled with water and inverted onto a Petri dish. Larvae migrating into the clean water in the Petri dish were collected using a pipette under a stereomicroscope (Archie and Ezenwa, 2011). Following isolation we prepared the larvae for DNA extraction by removing the outer sheath with a 0.15% sodium chlorite solution (Coles et al., 2006). Ex-sheathed larvae were then washed twice and stored in 95% ethanol until further analysis.
DNA was extracted from individual larvae following Archie and Ezenwa (2011). For each larva, we amplified then sequenced the ITS-2 region, an established marker for helminth identification (Heise et al., 1999; Zarlenga and Higgins, 2001), and then identified each specimen to species-level using reference samples in GENBANK. A total of 6–23 (mean = 10.2) larvae were sequenced from each of 30 buffalo. We identified four nematode species including: Cooperia oncophora, Haemonchus contortus, Haemonchus placei, and Trichostrongylus axei. To confirm the classification of Haemonchus sp., we examined the identity of 3 polymorphic nucleotides in the ITS-2 region known to differ between H. contortus and H. placei (Stevenson et al., 1995). To calculate the relative frequency of each parasite species per host we divided the number of larvae of each species by the total number of larvae identified. Egg abundance per species was estimated by multiplying the relative frequency of each species by the total egg count per sample (Wilson et al., 2008; Oliveira et al., 2009).
2.4. Statistical analysis
2.4.1. Aggregate parasite data: direct and indirect effects
We used general linear models to examine the direct costs of parasite co-infection on four distinct hematological parameters (see Table 1), and the association between parasite co-infection and host body condition. Separate analyses were performed for males and females since different sets of host traits were included in analyses for each sex. For males, the main predictor variables included two measures of infection, GI parasite richness and strongyle egg abundance. Since a number of factors can affect hematological status in buffalo (Beechler et al., 2009), we included the following variables: host age, season captured, and herd affiliation (nested within season) as covariates in our models. For female buffalo, we used the same measures of parasite infection as for males. Likewise, host age and season captured were included as covariates. We also included pregnancy status and lactation status. Since females were captured at two different sites (HIP and KNP), the site was included in these models to account for potential differences among locations in host condition and hematological parameters. For both male and female models, GI parasite richness was coded as an ordinal variable since only five parasite types were described. All other variables were continuous. Wilcoxon sign rank tests were used to compare GI parasite richness, strongyle intensity, hematological parameters, and body condition between males and females. For each sex, we ran independent models for body condition and each of four hematological parameters variables (hemoglobin (HG), hematocrit (HCT), red blood cell count (RBC), mean corpuscular volume (MCV)). Although the four hematological variables are correlated to differing degrees (Table S1), they have different biological interpretations and are therefore most informative when considered separately (Table 1). Residuals of all models were tested for normality with Shapiro-Wilk tests, and generalized linear models with an inverse Gaussian link function were used in all cases where model residuals were non-normal. When significant effects of GI parasite richness were detected, differences among levels of richness were examined further using Tukey’s multiple comparisons tests.
To examine the indirect effects of infection in terms of immune activation, lymphocytes were first examined as an independent variable in the hematology and condition models described above. Next, lymphocytes were examined as a dependent variable using general linear models with aggregate parasite richness, strongyle egg abundance, and the same covariates as described above for male and female models as explanatory variables. Although eosinophils also play a major role in anti-nematode defenses (Klion and Nutman, 2004), they were not associated with any hematological parameter in our preliminary analyses, and therefore were not included in our models.
2.4.2. Species-specific parasite data: direct and indirect effects
We identified four species of strongyle nematodes infecting our subset of 30 females (C. oncophora, H. placei, H. contortus, and T. axei). C. oncophora infected 96% of individuals; two Haemonchus sp., H. placei and H. contortus were the next most common infecting 44% and 24% of sampled buffalo, respectively; and T. axei was the most rare, infecting only one individual (4%). For the analyses, we categorized individuals based on the two predominant infection states: C. oncophora only infection (C-only) and C. oncophora-Haemonchus sp. co-infection (C–H). H. placei and H. contortus data were combined for the C–H infection group due to sample size (n = 3 C–H. contortus, n = 8 C–H. placei, and n = 2 C–Hp–Hc), and based on previous research suggesting these two species have very similar effects on host physiology (Le Jambre, 1995). We used this fine-scale parasite data to examine the effects of both co-infection status and species-specific egg abundance on host hematological profiles and associations with body condition. For both co-infection status and species-specific abundance analyses, individual host traits were not included as covariates in the models because of the relatively small number of individuals sampled for parasite identification (n = 30 females). However, because exploratory analyses suggested strong effects of pregnancy, but not season or age on response variables, five pregnant individuals were excluded from subsequent analysis.
To examine the effects of co-infection status on the host, we classified animals according to the number of strongyle nematode species harbored, and then used non-parametric Kruskal–Wallis tests to examine differences among uninfected individuals (n = 55 uninfected, non-pregnant females captured at the same study site) and individuals infected with C-only (n = 10) or C–H (n = 13). Next, we examined the relationships between Cooperia egg abundance and Haemonchus egg abundance and the same hematological parameters and condition indices. We included only individuals infected with at least one nematode species in the abundance analyses. For individuals infected with C-only, Haemonchus egg abundance was classified as zero. To examine the detectability of effects without knowing species identity, we also examined the relationships between total strongyle egg abundance and host hematology and body condition. All egg abundance data were log transformed to approach normality, and associations between egg abundance and performance indices were examined using linear regression models. Finally, to examine the indirect costs of infection, associations between total, Cooperia, and Haemonchus egg abundance and lymphocytes were explored using linear regression models. We also examined the costs of increased immune investment by testing associations between lymphocytes and residual hematological values or body condition score. Residual physiological indexes were calculated from the linear regression of total strongyle abundance versus RBC, HG, HCT, MCV, or body condition. For both species-specific and aggregate data, all statistical analyses were performed in R (Version 2.10.1, 2009) and SAS (Version 2.1, 2009).
3. Results
3.1. Aggregate parasite data
3.1.1. Sex differences in parasitism, hematological parameters, and condition
Males and females varied significantly in measures of parasitism and hematological parameters; these differences persisted if males were compared to only HIP females. At the aggregate level, parasite richness was higher among males (n = 203 males, 278 females, Wilcoxon’s W = 37,354, p < 0.00001, Fig. 1), and males shed significantly more strongyle eggs than females (W = 34,419, p < 0.00001). Females had higher HG, HCT, and MCV, but lower RBC than males (HG: W = 24,035, p = 0.0004; HCT: W = 23,831, p = 0.0003; MCV: W = 19,719, p < 0.00001; RBC: W = 33,694, p = 0.009). However, body condition did not differ between the sexes (W = 29,590, p = 0.62).
Figure 1.
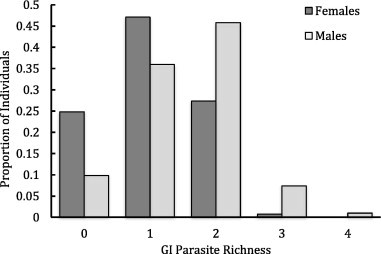
The proportion of male (n = 203) and female (n = 278) African buffalo infected with GI parasite communities of varying species richness.
3.1.2. Direct costs of infection
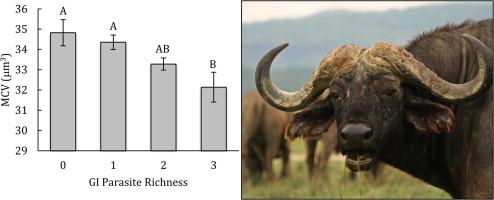
There were very few associations between aggregate parasite data and measures of hematological status and condition. All four hematological variables were strongly influenced by age, season, and herd affiliation in males (Table 2), and in females, site, season, and pregnancy had the most consistent effects on hematological parameters (Table 3). In males, body condition was significantly associated with season and herd (nested within season) (Table 2), while in females, season, site, pregnancy and lactation status all significantly influenced condition (Table 3). After controlling for covariates, we found that parasite richness was significantly and negatively associated with MCV in males (Table 2, Fig. 2), however, there was no correlation between richness and either RBC, HG, HCT or body condition (Table 2). In females, parasite richness was not a predictor of variation in condition or any of the four hematological variables (Table 3). In both males and females, no correlation was detected between strongyle intensity and body condition or hematological parameters.
Table 2.
Associations between hematological parameters and body condition index and individual host traits for male African buffalo (Syncerus caffer) from Hluhluwe-iMfolozi Park (n = 203).
| RBCa |
HG |
HCT |
MCVa |
Condition |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | χ2 | Est. | F | Est. | F | Est. | χ2 | Est. | χ2 |
| GI Parasite Richness | 5.15 | 1.13 | 1.01 | 11.5∗∗ | 0.59 | |||||
| Strongyle Abundance | 0.00001 | 0.94 | −0.00014 | 0.71 | −0.00001 | 0.81 | 0.00001 | 0.02 | 0.0002 | 0.67 |
| Lymphocytes | 0.0019 | 2.04 | −1.71 | 4.34* | −0.053 | 5.82* | 0.0001 | 1.38 | −1.03 | 0.76 |
| Age | 0.0002 | 6.16* | 0.13 | 13.4∗∗* | 0.0035 | 13.7∗∗∗ | −0.00001 | 37.1∗∗* | −0.026 | 0.24 |
| Season | −0.0036 | 78.7∗∗∗ | 1.86 | 91.1∗∗∗ | 0.038 | 51.7∗∗∗ | 0.0001 | 13.9∗∗∗ | 3.24 | 139∗∗∗ |
| Herd(season) | 39.3∗∗∗ | 5.92∗∗∗ | 6.47∗∗∗ | 30.8∗∗∗ | 3.07** | |||||
Significance level is indicated with asterisks: *<0.05, **<0.01, ∗∗∗<0.0001.
χ2 values from generalized linear models.
Table 3.
Associations between hematological parameters and body condition index and individual host traits for female African buffalo (Syncerus caffer) from Kruger National Park (KNP) and Hluhluwe-iMfolozi Park (HIP)(n = 278).
| RBCa |
HG |
HCT |
MCV |
Condition |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | χ2 | Est. | F | Est. | F | Est. | F | Est. | F |
| GI Parasite Richness | 7.8 | 2.15 | 0.29 | 1.09 | 1.71 | |||||
| Strongyle Abundance | −0.00001 | 0.07 | 0.0004 | 1.42 | 0.00004 | 0 | −0.00001 | 0 | −0.0004 | 0.51 |
| Lymphocytes | −0.0006 | 0.24 | −0.178 | 0.13 | −0.234 | 0.05 | −1.78 | 2.47 | 0.068 | 0 |
| Age | 0.0002 | 21.1∗∗∗ | −0.0007 | 0.1 | 0.067 | 1.8 | 0.393 | 55.3∗∗∗ | −0.121 | 7.4∗∗ |
| Pregnancy | −0.0003 | 0.68 | −0.68 | 16.5∗∗∗ | 1.13 | 9.28** | 1.66 | 17.9∗∗∗ | 1.3 | 15.5∗∗∗ |
| Lactation | 0.014 | 9.02** | −0.34 | 2.97 | 0.149 | 0.11 | 1.77 | 14.6∗∗∗ | −2.12 | 29.6∗∗* |
| Season (dry) | 0.0018 | 23.5∗∗∗ | −1 | 42.1∗∗* | 0.131 | 0.15 | 1.33 | 13.6∗∗∗ | −5.58 | 335∗∗∗ |
| Site (HIP) | −0.0036 | 65.7∗∗* | 2.97 | 266∗∗∗ | −32.5 | 6444∗∗∗ | −1.27 | 8.86** | −1.09 | 9.12** |
Significance level is indicated with asterisks: *<0.05; **<0.01; ∗∗∗<0.0001.
χ2 values from a generalized linear model.
Figure 2.
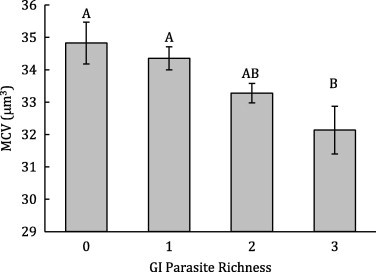
The relationship between gastrointestinal parasite richness and MCV for male buffalo. Least squares means ± 1 standard error are shown. Letters designate significant differences among levels of GI parasite richness.
3.1.3. Indirect costs of infection
When we examined how parasite infection influenced adaptive immune function in males, we found that strongyle egg abundance, but not GI parasite richness, was significantly and positively associated with lymphocyte count after controlling for key covariates (egg abundance: estimate = 0.00004, F1,202 = 8.07, p = 0.005; parasite richness: F3,202 = 0.19, p = 0.91; age: estimate = −0.019, F1,202 = 43.7, p < 0.0001; herd (nested within season): F8,202 = 2.00, p = 0.049; season (dry): estimate = 0.035, F1,202 = 2.55, p = 0.11). However, for females, there was no association between either parasite measure and lymphocytes (egg abundance: estimate = 0.00004, F1,277 = 1.20, p = 0.27; parasite richness: F2,277 = 0.17, p = 0.92; age: estimate = −0.014, F1,277 = 25.9, p < 0.0001; pregnancy: estimate = −0.030, F1,277 = 2.08, p = 0.15; lactation: estimate = 0.005, F1,277 = 0.04, p = 0.84; site: estimate (HIP) = 0.078, F1,277 = 12.0, p = 0.0006; season: estimate (dry) = −0.055, F1,277 = 8.17, p = 0.005). When we examined the potential effects of lymphocytes on host hematological status and condition, we found that lymphocytes were significantly and negatively associated with HG and HCT, but not RBC, MCV, or body condition in males (Table 2). Once again, there was no association between lymphocytes and any physiological parameter in females (Table 3).
3.2. Species specific data
3.2.1. Direct costs of infection
Co-infection status had no detectable effects on hematological variables. Comparing uninfected to C-only and C–H infected females, we found no differences among groups for RBC, HG, HCT, or MCV (RBC: χ2 = 2.76, p = 0.25; HG: χ2 = 5.68, p = 0.058; HCT: χ2 = 2.03, p = 0.36; MCV: χ2 = 3.86, p = 0.15). However, infection status was significantly associated with body condition, with C–H co-infected females being in significantly poorer condition than uninfected and C-only infected females (χ2 = 9.24, p = 0.010, Fig. 3).
Figure 3.
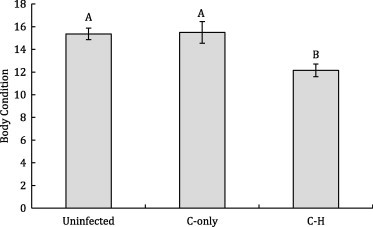
Body condition was lower in Cooperia-Haemonchus co-infected buffalo compared to uninfected and Cooperia-only singly-infected buffalo. Means ± 1 standard error are shown. Letters denote significant differences among groups.
When we analyzed the effects of total and species-specific egg abundance on hematological profile and body condition we found a series of interesting patterns. Across all individuals (n = 23), total strongyle egg abundance was negatively correlated with HCT (r = −0.50, p = 0.015; Fig. 4), but had no detectable effect on RBC, HG or MCV (RBC: r = −0.06, p = 0.77; HG: r = −0.16, p = 0.46; MCV: r = −0.40, p = 0.06; Fig. 4, Column 1). When we subdivided total egg abundance by species, we found that C. oncophora egg abundance was negatively associated with HCT and MCV (HCT: r = −0.42, p = 0.04; MCV: r = −0.45, p = 0.03, Column 2). However, C. oncophora egg abundance was not significantly correlated with either RBC or HG (RBC: r = 0.03, p = 0.87; HG: r = −0.05, p = 0.82; Fig. 4, Column 2). On the other hand, Haemonchus sp. egg abundance was significantly and negatively correlated with RBC count and HG (RBC: r = −0.46, p = 0.03; HG: r = −0.41, p = 0.048; Fig. 4, Column 3), but there was no significant association between either HCT or MCV and Haemonchus sp. egg abundance (HCT: r = −0.26, p = 0.23; MCV: r = 0.33, p = 0.12; Fig. 4, Column 3). Overall, we found that each nematode species showed strong negative effects on two separate hematological indices (Cooperia: HCT and MCV; Haemonchus: HG and RBC), but when considered in aggregate (i.e. total egg abundance), effects were only detectable for a single index (HCT). Similarly, associations with body condition were not detectable when we tested the strength of the relationships between total strongyle egg abundance and condition (r = 0.38, p = 0.077; Fig. 4). However, when the species were considered separately, body condition was negatively correlated with Haemonchus sp. egg abundance (r = −0.55, p = 0.007), and positively correlated with C. oncophora egg abundance (r = 0.52, p = 0.011; Fig. 4).
Figure 4.
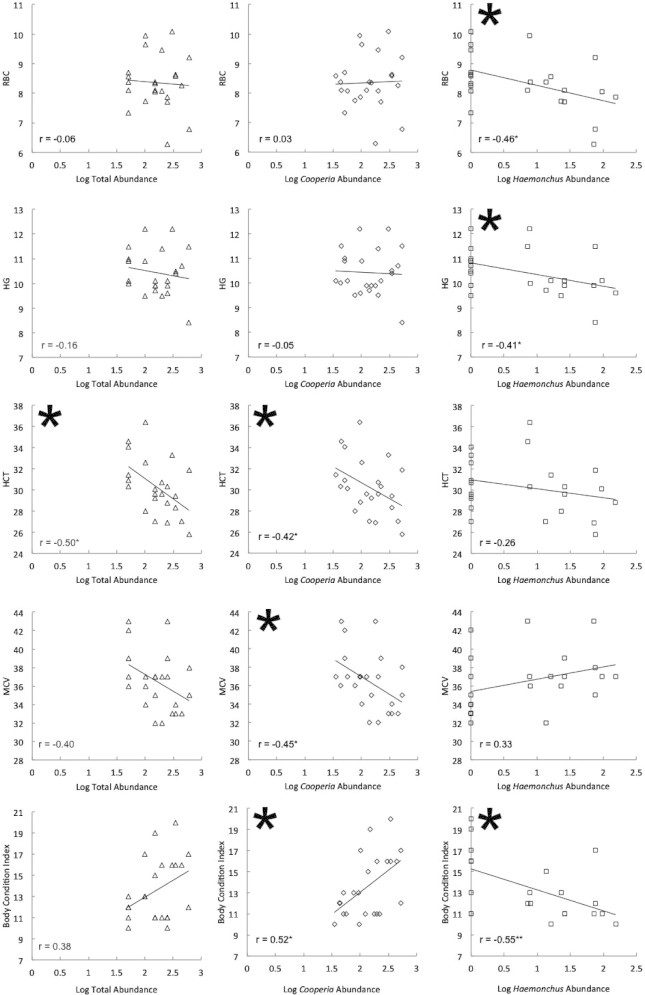
The relationship between total and species-specific strongyle egg abundance and four hematological parameters: RBC (cells/uL × 106), HG (g/dL), HCT (%), MCV(10−15L), and body condition for 23 female African buffalo. If total strongyle egg abundance was considered (left column), a significant correlation was detected between egg abundance and HCT. HCT, MCV, and body condition index were significantly correlated with Cooperia egg abundance (center column). HG, RBC, and body condition index were significantly correlated with Haemonchus egg abundance (right column). Significance is denoted by a large asterisk, and correlation coefficients (r) are displayed with significance level (•<0.05, **<0.01).
3.2.2. Indirect costs of infection
In terms of indirect effects, investment in lymphocytes did not differ among uninfected, C-only infected, and C–H co-infected females (χ2 = 4.04, p = 0.13). However, lymphocyte counts were positively correlated with C. oncophora egg abundance (r = 0.55, p = 0.006) and total strongyle egg abundance (r = 0.49, p = 0.018), but there was no association between lymphocytes and Haemonchus egg abundance (r = −0.28, p = 0.19; Fig. 5). In addition, costs of lymphocyte investment were detected with MCV. Lymphocyte investment was also negatively correlated with body condition. Accounting for total strongyle egg abundance, residual MCV was negatively correlated with lymphocyte investment (r = −0.45, p = 0.031, Fig. 5), while residual body condition was positively correlated with lymphocyte investment (r = 0.44, p = 0.038, Fig. 5). There was no association between residual RBC, HG, or HCT and lymphocyte investment (RBC: r = 0.38, p = 0.07, HG: r = 0.21, p = 0.34, HCT: r = 0.09, p = 0.68).
Figure 5.
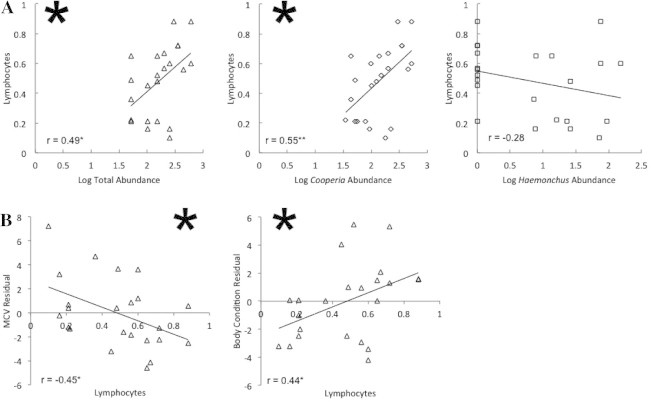
Indirect costs of infection demonstrated by the relationships between total and species-specific strongyle egg abundance and lymphocyte investment for 23 female African buffalo (A). After accounting for total strongyle intensity, lymphocyte investment was significantly and negatively correlated with MCV, and positively correlated with body condition (B). Significance is denoted by a large asterisk. The correlation coefficient (r) is noted with significance level (•<0.05, **<0.01).
4. Discussion
Our results suggest that the costs of parasitism are detectable over the short term using physiological performance indices. Specifically, host hematological profiles appear to be sensitive to variation in infection levels, with parasite species richness and identity largely driving observed direct effects. In addition, we found that indirect costs of infection, due to investment in immunological defenses, were detectable via changes in host hematological parameters. Importantly, our data show that the detectability of both direct and indirect effects depended on the resolution of the parasite data. With aggregated parasite data, physiological effects may only emerge when effect sizes are considerably large, which may explain the differences we found in male compared to female buffalo. Our results also suggest that aggregated data can mask parasite effects because even closely related parasite species may alter host hematological parameters and immunity in different ways and to differing degrees.
4.1. Aggregate parasite data: direct and indirect effects
With aggregated parasite data, the direct costs of co-infection were only detectable for males in our study; we found no association between measures of parasitism and host hematological variables in females. In males, GI parasite richness was significantly and negatively correlated with the mean corpuscular volume (MCV) of red blood cells. This pattern of variation in MCV may be indicative of young red blood cells and increased RBC turnover due to blood loss (Jain, 1993; Feldman et al., 2000). Variation in RBC turnover has previously been linked to both individual survival and reproduction in wild populations (Noyce and Garshelis, 1994; Bearhop et al., 1999; Harvey, 2000). Interestingly, our models show that it was species richness rather than egg abundance that had a significant effect on MCV; and even without knowing parasite community composition, we detected a stepwise decrease in MCV with the number of parasite types. The diversity of the parasite community within an individual host may therefore be an important, yet underappreciated, indicator of the potential magnitude of parasite-induced costs. Similarly, stepwise effects of helminth species richness on host hemoglobin levels have often been detected in human populations (Ezeamama et al., 2005, 2008; Midzi et al., 2010). These studies support the idea that parasite community richness itself, separate from the intensity of infection, has physiological costs. The costs associated with parasite richness may be particularly potent because the richness of different parasite groups commonly co-vary (Krasnov et al., 2005; Balestrieri et al., 2006; Holmstad et al., 2008). Furthermore, the tendency for particular hosts to have multiple parasite infections may create positive feedback cycles that magnify the net parasite effects on these hosts.
Our aggregate parasite data also show that males experienced greater indirect costs of infection than females. Specifically, lymphocyte counts were negatively correlated with hemoglobin (HG) and hematocrit (HCT) in males, even after accounting for GI parasite richness and strongyle egg abundance. By contrast, there was no effect of lymphocytes on any hematological parameter in females. Males may therefore pay a cost of investing in lymphocytes since low HG and HCT levels are often associated with lower aerobic performance, reproduction, and survival in mammals (Gledhill et al., 1999; Allen, 2000). Evidence from livestock and human medicine suggests that the potential fitness costs of declines in HG and HCT can be substantial. For example, Aumont et al. (1991) found that helminth infected calves had a mean HCT approximately 3% lower than uninfected calves, and also gained 10–15% less weight per day over a 7 month period. Similarly, lower HG levels due to helminth infection have been linked to stunted growth and reduced aerobic performance in human children (Bustinduy et al., 2011). In general, the complex and dynamic nature of the vertebrate immune system makes the fitness costs of immune system maintenance and use difficult to assess, particularly in wild populations (Lochmiller and Deerenberg, 2000; Martin et al., 2008). However, lymphocytes are energetically costly to develop and maintain (Klasing, 2004), play a major role in helminth defense (Janeway, 2008), and have been shown to correlate with helminth diversity across mammal species (Bordes and Morand, 2009). As such, our results linking lymphocytes to host hematological profiles suggest that the costs of immune deployment may be quantifiable by examining changes in other hematological components, and may offer a potentially new approach for assessing the costs of immunity.
The male biased direct and indirect costs of infection we observed at the aggregate level may be a function of male-biased infection. In our study population, males had 50% richer GI parasite communities than females, and this may have led to stronger, and therefore more detectable, costs than in females. Male biased parasitism is common in mammals, particularly for arthropod and helminth parasites and in host species with a high degree of sexual size dimorphism (Poulin, 1996; Schalk and Forbes, 1997; Moore and Wilson, 2002). Furthermore, sex-biases in infection have been linked to biases in parasite-related fitness costs both within and across host species (Gulland, 1992; Moore and Wilson, 2002; Craig et al., 2009). For this reason, sex-biased infection may help account for the difference in our ability to detect direct hematological costs and indirect immune-mediated costs of parasitism in male versus female buffalo using aggregate parasite data.
4.2. Species-specific parasite data: direct and indirect effects
Although no costs of infection were detected in females using aggregate parasite data, significant direct effects emerged once the abundance of individual parasite species was taken into account. In particular, we found that Cooperia egg abundance was negatively correlated with MCV and HCT, while Haemonchus egg abundance was negatively correlated with HG and RBC. The known effects of Cooperia and Haemonchus infection in livestock help provide biological explanations for the patterns we observed in free-ranging buffalo. In livestock infected with Cooperia or other non-bloodsucking nematodes, impaired red blood cell regeneration has also been described and is believed to be a consequence of impaired digestion and resulting deficiencies in key nutrients, as well as the costs of immune responses to infection (Baker and Douglas, 1957, 1966; Soulsby, 1982; Feldman et al., 2000). For Cooperia-infected buffalo, associations between egg abundance, HCT and MCV may indicate impaired oxygen carrying capacity and red blood cell regeneration (Jain, 1993). Haemonchus is known to cause anemia, emaciation, and even mortality in livestock (Fourie, 1931; Le Jambre, 1995; Yacob et al., 2008). In buffalo, the associations between Haemonchus egg abundance, HG and RBC are likely indicative of chronic blood loss and associated costs, including reduced oxygen binding capacity and reduced aerobic capacity (Jain, 1993; Bustinduy et al., 2011).
In contrast to the hematological effects described above, the associations we found between parasite egg abundance and host body condition were less clear cut. Haemonchus egg abundance was negatively correlated with body condition, while Cooperia abundance was positively correlated with condition. These contradicting relationships mirrored our analysis showing that body condition did not differ between uninfected and C-only infected individuals, but was significantly lower for C–H infected individuals. The apparent positive correlation between Cooperia egg abundance and buffalo body condition runs counter to observations in livestock linking this parasite to declines in growth and production (Stromberg et al., 2012). Further work in our study system is needed to determine the factors underlying the positive and negative correlations we observed between body condition and Cooperia and Haemonchus egg abundance, respectively.
The indirect costs of infection also varied by parasite species in our study. Cooperia abundance was significantly and positively correlated with lymphocytes, while Haemonchus was not associated with this measure of immune defense. Since Cooperia infection is known to induce strong B-cell responses in livestock (Nieuwland et al., 1995; Parmentier et al., 1995), it seems plausible that in buffalo Cooperia may also induce a strong B-cell response, which likely corresponds to an increase in circulating lymphocytes. By contrast, two recent livestock studies found that Haemonchus intensity was negatively correlated with lymphocytes (Lacroux et al., 2006; Rowe et al., 2008). Our analysis of indirect costs also revealed a negative correlation between lymphocytes and residual MCV (i.e. MCV corrected for strongyle egg abundance), indicating that investment in lymphocyte defenses may carry a physiological cost detectable by examining red blood cell volume (MCV). Since Cooperia egg abundance was directly and negatively correlated with MCV, these patterns collectively support the idea that in buffalo the costs of non-blood sucking nematodes like Cooperia may manifest as by-products of immune investment. However, further research is needed to determine the directionality of the lymphocyte-MCV relationship we observed (i.e. lymphocytes affecting MCV rather than red blood cell regeneration influencing lymphocyte numbers). Interestingly, we also found that body condition corrected for strongyle egg abundance (i.e. residual body condition) was positively correlated with lymphocyte investment, possibly indicating that those individuals able to maintain high body condition for their level of Cooperia infection may also able to sustain high lymphocyte defenses. Nevertheless, the same animals may still suffer the indirect costs of high lymphocyte investment as reductions in MCV.
Given that Cooperia and Haemonchus parasites had different direct and indirect effects on host hematology, it is perhaps not surprising that many of these species-specific effects were masked when total strongyle egg abundance was considered. In fact, overall egg abundance was significantly correlated with only one hematological index, HCT. This may be because the effects of both parasites on HCT were qualitatively in the same direction (i.e. negative effects), whereas for several other parameters, the neutral effect of one parasite may have swamped out the negative effect of the other (Fig. 4). In general, our analyses accounting for parasite species identity demonstrate that the costs of infection may be difficult to detect when co-infecting species are considered in aggregate. Reciprocally, the physiological costs associated with individual parasite species may be difficult to assess in the wild due to the presence of co-infecting parasites. Although in our study we could not fully distinguish the effects of each species from co-infection effects since we did not observe any Haemonchus-only infections, we were able to infer species-specific costs based on egg abundance data. These species-specific costs provide insight as to why we failed to detect any direct or indirect effects of parasites on females in our aggregate analyses. Thus, it is important to consider the effects of both parasite species identity and parasite community composition when assessing parasite-mediated fitness costs.
4.3. Synthesis
Although it is fairly well-established that different GI helminths have distinct effects on livestock hosts (Stear et al., 1998; Bowman, 2009), there is still very little known about how individual species within the GI parasite community combine to contribute to the costs of infection in wildlife species. This difference between livestock and wildlife studies arises in large part because wildlife sampling is often non-invasive with researchers using fecal samples to characterize and quantify GI helminth infection (Wimmer et al., 2004; Turner and Getz, 2010; McLean et al., 2012). Determining helminth community composition and relative species abundance from fecal samples is a substantial challenge. Morphological based methods for identifying immature stages of helminth parasites (e.g. eggs and larvae) are not always feasible or are extremely time consuming since differences among species are often minute (Keith, 1953; Van Wyk et al., 2004). More recently, molecular techniques have been used to identify both helminth eggs and larvae (Zarlenga et al., 1998; Zarlenga and Higgins, 2001; Wimmer et al., 2004; Bott et al., 2009). In this study we used third stage larvae (L3) for genetic identification because they contain more DNA than eggs and are easier to separate from fecal inhibitors (Gasser et al., 2008). However, the use of larvae instead of eggs may have biased our species composition results due to differential egg viability and larval survival during culture (Berrie et al., 1988, Dobson et al., 1992, but see Bryan and Kerr, 1989). Nevertheless, our use of a combination of genetic-based identification and traditional egg counts revealed strong patterns that were not detectable with either data set alone. For instance, while there was no relationship between MCV and total strongyle abundance based on traditional egg count data, and no difference in MCV between uninfected and Cooperia-infected females based on genetic data, when these datasets were combined a strong negative relationship between Cooperia egg abundance and MCV emerged (Fig. 4). These results highlight a practical way in which traditional egg count data can be combined with molecular data to address key questions about helminth co-infection in wildlife populations where invasive sampling is not feasible.
Our study suggests that parasites impose both direct and indirect costs on their hosts that are detectable using hematological indices. We detected more interactions between GI parasite infection and hematological parameters than with a traditional measure of energetic reserves, a body condition index based on fat storage. In males, we failed to detect any effects of infection on body condition at the aggregate parasite level, although we detected both direct and indirect effects of infection using hematological indices. In our species-specific analysis for females, when parasite species were analyzed in combination we detected parasite-mediated costs for at least one hematological variable (HCT), but not for body condition. On the other hand, combining species-specific nematode data and egg abundance data revealed strong direct costs of infection on all four hematological variables and body condition as well as indirect immune-mediated costs.
The presence of consistent negative associations between hematological indices and egg abundance, and parasite-specific patterns, both support the idea that our results are indicative of parasite effects of the host and not effects of the host on the parasites. Nevertheless, experimental data will be required to establish a definitive, causal link between helminth infection and host hematology. In terms of body condition, species-specific parasite analyses revealed both positive and negative association patterns depending on the parasite, making it difficult to interpret condition effects. These results highlight the difficulty inherent in interpreting parasite-body condition correlations, especially if factors that increase body condition also enhance parasite exposure (e.g. foraging, Hutchings et al., 2000), or if the effects of parasites on body condition cannot be distinguished from the effects on condition on parasite susceptibility. Taken together, our findings suggest that hematological indices may be a more flexible and sensitive tool for assessing the complex relationships among parasites, immune function, and host energy stores than fat-based condition indices. For this reason, hematological profiles may represent a useful tool for assessing the costs of GI parasite infection in wild populations.
Acknowledgements
We thank Kwazulu-Natal Wildlife Service, Kwazulu-Natal State Veterinary Service, and South Africa National Parks Veterinary Services for conducting the buffalo capture operations. For assistance in the field and laboratory, we thank R. Spaan, J. Calldo, N. Armour, D. Cooper, A. McCall, W. McCall, C. Reed, S. Ras, S. van Rensburg, J. Britt, F. Gardipee, K. Kanapeckas, M. Matokazi and M. O’Brien. Animal protocols used in this study were approved by the University of Montana (AUP #: 027-05VEDBS-082205) and Oregon State University (ACUP # 3267) Animal Care and Use Committees. This work was supported by a National Science Foundation Ecology of Infectious Diseases Grant (DEB-1102493/EF-0723928, EF-0723918) and Small Grant for Exploratory Research (DEB-0541762, DEB-0541981).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Sarah A. Budischak, Email: sabudi@uga.edu.
Anna E. Jolles, Email: jollesa@science.oregonstate.edu.
Vanessa O. Ezenwa, Email: vezenwa@uga.edu.
Appendix A. Supplementary data
References
- Allen L.H. Anemia and iron deficiency: effects on pregnancy outcome. Am. J. Clin. Nutr. 2000;71:1280S–1284S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- Archie E.A., Ezenwa V.O. Population genetic structure and history of a generalist parasite infecting multiple sympatric host species. Int. J. Parasitol. 2011;41:89–98. doi: 10.1016/j.ijpara.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Aumont G., Gauthier D., Coulaud G., Gruner L. Gastro-intestinal parasitism of cattle in native pasture grazing system in Guadelupe (French West Indies) Vet. Parasitol. 1991;40:29–46. doi: 10.1016/0304-4017(91)90081-6. [DOI] [PubMed] [Google Scholar]
- Baker N.F., Douglas J.R. The pathogenesis of trichostrongyloid parasites: ferrokinetic studies in ruminants. Am. J. Vet. Res. 1957;18:295–302. [PubMed] [Google Scholar]
- Baker N.F., Douglas J.R. Blood alterations in helminth infection. In: Soulsby E.J.L., editor. Biology of Parasites: Emphasis on Veterinary Parasites, World Association for the Advancement of Veterinary Parasitology. Academic Press; New York: 1966. pp. 155–183. [Google Scholar]
- Balestrieri A., Remonti L., Ferrari N., Ferrari A., Lo Valvo T., Robetto S., Orusa R. Sarcoptic mange in wild carnivores and its co-occurrence with parasitic helminths in the Western Italian Alps. Eur. J. Wildl. Res. 2006;52:196–201. [Google Scholar]
- Bearhop S., Griffiths R., Orr K., Furness R.W. Mean corpuscular volume (MCV) as a measure of condition in birds. Ecol. Lett. 1999;2:352–356. [Google Scholar]
- Beechler B.R., Jolles A.E., Ezenwa V.O. Evaluation of hematologic values in free-ranging African buffalo (Syncerus caffer) J. Wildl. Dis. 2009;45:57–66. doi: 10.7589/0090-3558-45.1.57. [DOI] [PubMed] [Google Scholar]
- Behnke J.M., Gilbert F.S., Abu-Madi M.A., Lewis J.W. Do the helminth parasites of wood mice interact? J. Anim. Ecol. 2005;74:982–993. [Google Scholar]
- Berger L., Speare R., Daszak P., Green D.E., Cunningham A.A., Goggin C.L., Slocombe R., Ragan M.A., Hyatt A.D., McDonald K.R., Hines H.B., Lips K.R., Marantelli G., Parkes H. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrie D.A., East I.J., Bourne A.S., Bremmer K.C. Differential recoveries from faecal cultures of larvae of some gastro-intestinal nematodes of cattle. J. Helminthol. 1988;62:110–114. doi: 10.1017/s0022149x00011330. [DOI] [PubMed] [Google Scholar]
- Bordes F., Morand S. Coevolution between multiple helminth infestations and basal immune investment in mammals: cumulative effects of polyparasitism? Parasitol. Res. 2009;106:33–37. doi: 10.1007/s00436-009-1623-6. [DOI] [PubMed] [Google Scholar]
- Bott N.J., Campbell B.E., Beveridge I., Chilton N.B., Rees D., Hunt P.W., Gasser R.B. A combined microscopic-molecular method for the diagnosis of strongylid infections in sheep. Int. J. Parasitol. 2009;39:1277–1287. doi: 10.1016/j.ijpara.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Bowman D.D. ninth ed. Saunders Elsevier; St. Louis, MO: 2009. Georgis’ Parasitology for Veterinarians. [Google Scholar]
- Bryan R.P., Kerr J.D. The relation between the natural worm burden on steers and the fecal egg count differentiated to species. Vet. Parasitol. 1989;30:327–334. doi: 10.1016/0304-4017(89)90102-7. [DOI] [PubMed] [Google Scholar]
- Bustinduy A.L., Thomas C.L., Fiutem J.J., Parraga I.M., Mungai P.L., Muchiri E.M., Mutuku F., Kitron U., King C.H. Measuring fitness of Kenyan children with polyparasitic infections using the 20-meter shuttle run test as a morbidity metric. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambellan A., Chailleux E., Similowski T., Grp A.O. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest. 2005;128:1201–1208. doi: 10.1378/chest.128.3.1201. [DOI] [PubMed] [Google Scholar]
- Chen M.H., Chang P.M.H., Chen P.M., Tzeng C.H., Chu P.Y., Chang S.Y., Yang M.H. Prognostic significance of a pretreatment hematologic profile in patients with head and neck cancer. J. Cancer Res. Clin. Oncol. 2009;135:1783–1790. doi: 10.1007/s00432-009-0625-1. [DOI] [PubMed] [Google Scholar]
- Colditz I.G. Six costs of immunity to gastrointestinal nematode infections. Parasite Immunol. 2008;30:63–70. doi: 10.1111/j.1365-3024.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- Coles G.C., Jackson F., Pomroy W.E., Prichard R.K., von Samson-Himmelstjerna G., Silvestre A., Taylor M.A., Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006;136:167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Combes C. The University of Chicago Press; Chicago: 2001. Parasitism: The Ecology and Evolution of Intimate Interactions. [Google Scholar]
- Craig B.H., Tempest L.J., Pilkington J.G., Pemberton J.M. Metazoan-protozoan parasite co-infections and host body weight in St Kilda Soay sheep. Parasitology. 2008;135:433–441. doi: 10.1017/S0031182008004137. [DOI] [PubMed] [Google Scholar]
- Craig B.H., Jones O.R., Pilkington J.G., Pemberton J.M. Re-establishment of nematode infra-community and host survivorship in wild Soay sheep following anthelmintic treatment. Vet. Parasitol. 2009;161:47–52. doi: 10.1016/j.vetpar.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Dobson R.J., Barnes E.H., Birclijin S.D., Gill J.H. The survival of Ostertagia circumcincta and Trichostrongylus colubriformis in faecal culture as a source of bias in apportioning egg counts to worm species. Int. J. Parasitol. 1992;22:1005–1008. doi: 10.1016/0020-7519(92)90060-x. [DOI] [PubMed] [Google Scholar]
- Ezeamama A.E., Friedman J.F., Olveda R.M., Acosta L.P., Kurtis J.D., Mor V., McGarvey S.T. Functional significance of low-intensity polyparasite helminth infections in anemia. J. Infect. Dis. 2005;192:2160–2170. doi: 10.1086/498219. [DOI] [PubMed] [Google Scholar]
- Ezeamama A.E., McGarvey S.T., Acosta L.P., Zierler S., Manalo D.L., Wu H.W., Kurtis J.D., Mor V., Olveda R.M., Friedman J.F. The synergistic effect of concomitant schistosomiasis, hookworm, and Trichuris infections on children’s anemia burden. PLoS Negl. Trop. Dis. 2008;2 doi: 10.1371/journal.pntd.0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezenwa V.O. Habitat overlap and gastrointestinal parasitism in sympatric African bovids. Parasitology. 2003;126:379–388. doi: 10.1017/s0031182002002913. [DOI] [PubMed] [Google Scholar]
- Ezenwa V.O., Jolles A.E. Horns honestly advertise parasite infection in male and female African buffalo. Anim. Behav. 2008;75:2013–2021. [Google Scholar]
- Ezenwa V.O., Jolles A.E., O’Brian M.P. A reliable body condition scoring technique for estimating condition in African buffalo. Afr. J. Ecol. 2009;49:476–481. [Google Scholar]
- Feldman B.V., Zinkl J.G., Jain N.C. fivth ed. Lippincott, Williams & Wilkins; Philadelphia: 2000. Schalm’s Veterinary Hematology. [Google Scholar]
- Fourie, P.J.J., 1931. The hematology and pathology of haemonchosis in sheep. Union of South Africa Department of Agriculture, Division of Veterinary Services and Animal Industry Annual Report 17, p. 495.
- Gasser R.B., Bott N.J., Chilton N.B., Hunt P., Beveridge I. Toward practical, DNA-based diagnostic methods for parasitic nematodes of livestock – bionomic and biotechnological implications. Biotechnol. Adv. 2008;26:325–334. doi: 10.1016/j.biotechadv.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Gill N., Shaikh A.A., Khan M.M., Memon M.S. Influence of intestinal cestodes on the blood picture of the brown rats (Rattus norvegicus) of Hyderabad Sindh, Pakistan. Pak. J. Biol. Sci. 2007;10:4479–4484. doi: 10.3923/pjbs.2007.4479.4484. [DOI] [PubMed] [Google Scholar]
- Gledhill N., Warburton D., Jamnik V. Haemoglobin, blood volume, cardiac function, and aerobic power. Can. J. Appl. Physiol.: Rev. Can. Physiol. Appl. 1999;24:54–65. doi: 10.1139/h99-006. [DOI] [PubMed] [Google Scholar]
- Gulland F.M.D. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology. 1992;105:493–503. doi: 10.1017/s0031182000074679. [DOI] [PubMed] [Google Scholar]
- Harmening D.M. third ed. F.A. Davis Company; Philadelphia: 1997. Clinical Hematology and Fundamentals of Hemostasis. p. 743. [Google Scholar]
- Harvey J.W. Microcytic anemias. In: Feldman B.V., Zinkl J.G., Jain N.C., editors. Schalm’s Veterinary Hematology. fivth ed. Narayana Press; Denmark: 2000. pp. 200–204. [Google Scholar]
- Heise M., Epe C., Schieder T. Differences in the second international transcribed spacer (ITS-2) of eight species of gastrointestinal nematodes of ruminants. J. Parasitol. 1999;85:431–435. [PubMed] [Google Scholar]
- Holmstad P.R., Jensen K.H., Skorping A. Ectoparasite intensities are correlated with endoparasite infection loads in willow ptarmigan. Oikos. 2008;117:515–520. [Google Scholar]
- Hudson P.J., Dobson A.P., Newborn D. Prevention of population cycles by parasite removal. Science. 1998;282:2256–2258. doi: 10.1126/science.282.5397.2256. [DOI] [PubMed] [Google Scholar]
- Hutchings M.R., Kyriazakis I., Papachristou T.G., Gordon I.J., Jackson F. The herbivores’ dilemma: trade-offs between nutrition and parasitism in foraging decisions. Oecologia. 2000;124:242–251. doi: 10.1007/s004420000367. [DOI] [PubMed] [Google Scholar]
- Jain N.C. Lea & Febiger; Philadelphia: 1993. Essentials of Veterinary Hematology. p. 417. [Google Scholar]
- Janeway C. seventh ed. Garland Science; New York, NY: 2008. Janeway’s Immunobiology. [Google Scholar]
- Jennings J.B., Calow P. The relationship between high fecundity and the evolution of entoparasitism. Oecologia. 1975;21:109–115. doi: 10.1007/BF00345553. [DOI] [PubMed] [Google Scholar]
- Jolles A.E., Cooper D.V., Levin S.A. Hidden effects of chronic tuberculosis in African buffalo. Ecology. 2005;86:2258–2264. [Google Scholar]
- Keith R.K. The differentiation of the infective larvae of some common nematode parasites of cattle. Aust. J. Zool. 1953;12:223–235. [Google Scholar]
- Klasing K.C. The costs of immunity. Acta Zool. Sin. 2004;50:961–969. [Google Scholar]
- Klion A.D., Nutman T.B. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- Krasnov B.R., Mouillot D., Khokhlova I.S., Shenbrot G.I., Poulin R. Covariance in species diversity and facilitation among non-interactive parasite taxa: all against the host. Parasitology. 2005;131:557–568. doi: 10.1017/S0031182005007912. [DOI] [PubMed] [Google Scholar]
- Lacroux C., Nguyen T., Andreoletti O., Prevot F., Grisez C., Bergeaud J.P., Brunel J., Francois D., Dorchies P., Jacquiet P. Haemonchus contortus (Nematoda: Trichostrongylidae) infection in lambs elicits and unequivocal Th2 immune response. Vet. Res. 2006;37 doi: 10.1051/vetres:2006022. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D., Kuris A.M. Parasitic castration: the evolution and ecology of body snatchers. Trends Parasitol. 2009;25:564–572. doi: 10.1016/j.pt.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Le Jambre L.F. Relationship of blood loss to worm numbers, biomass, and egg production in Haemonchus infected sheep. Int. J. Parasitol. 1995;25:269–273. doi: 10.1016/0020-7519(94)00118-8. [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L., Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Lotfollahzadeh S., Mohri M., Bahadori S.R., Dezfouly M.R.M., Tajik R. The relationship between normocytic, hypochromic anaemia and iron concentration together with hepatic enzyme activities in cattle infected with Fasciola hepatica. J. Helminthol. 2008;82:85–88. doi: 10.1017/S0022149X07874232. [DOI] [PubMed] [Google Scholar]
- Martin L.B., Weil Z.M., Nelson R.J. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos. Trans. R. Soc. B: Biol. Sci. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean E.R., Kinsella J.M., Chiyo P., Obanda V., Moss C., Archie A.E. Genetic identification of five strongyle nematode parasites in wild African elephants (Loxodonta africana) J. Wildl. Dis. 2012;48:707–716. doi: 10.7589/0090-3558-48.3.707. [DOI] [PubMed] [Google Scholar]
- Midzi N., Mtapuri-Zinyowera S., Mapingure M.P., Sangweme D., Chirehwa M.T., Brouwer K.C., Mudzori J., Hlerema G., Mutapi F., Kumar N., Mduluza T. Consequences of polyparasitism on anaemia among primary school children in Zimbabwe. Acta Trop. 2010;115:103–111. doi: 10.1016/j.actatropica.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Moore I.T., Hopkins W.A. Interactions and trade-offs among physiological determinants of performance and reproductive success. Integr. Comp. Biol. 2009;49:441–451. doi: 10.1093/icb/icp081. [DOI] [PubMed] [Google Scholar]
- Moore S.L., Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. [DOI] [PubMed] [Google Scholar]
- Nieuwland M.G., Ploeger H.W., Kloosterman A., Parmentier H.K. Systemic antibody responses of calves to low molecular weight Cooperia oncophora antigens. Vet. Parasitol. 1995;59 doi: 10.1016/0304-4017(94)00754-z. [DOI] [PubMed] [Google Scholar]
- Noyce K.V., Garshelis D.L. Body size and blood characteristics as indicators of condition and reproductive performance in black bears. Bears Biol. Manage. 1994;9:481–496. [Google Scholar]
- Oliveira M.C.S., Alencar M.M., Chagas A.C.S., Giglioti R., Oliveira H.N. Gastrointestinal nematode infection in beef cattle of different genetic groups in Brazil. Vet. Parasitol. 2009;166:249–254. doi: 10.1016/j.vetpar.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Parmentier H.K., Ploeger H.W., Nieuwland M.G., Souren P.J., Van Pinxteren L.A., Rietveld F.W., De Vries Reilingh G., Kloosterman A. Low molecular weight Cooperia oncophora antigens: characterization and humoral immune responses in calves mono-infected with 100,000 infective larvae. Vet. Parasitol. 1995;59 doi: 10.1016/0304-4017(94)00753-y. [DOI] [PubMed] [Google Scholar]
- Petney T.N., Andrews R.H. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int. J. Parasitol. 1998;28:377–393. doi: 10.1016/s0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- Pfafle M., Petney T., Elgas M., Skuballa J., Taraschewski H. Tick-induced blood loss leads to regenerative anaemia in the European hedgehog (Erinaceus europaeus) Parasitology. 2009;136:443–452. doi: 10.1017/S0031182009005514. [DOI] [PubMed] [Google Scholar]
- Poulin R. Sexual inequalities in helminth infections: A cost of being male? Am. Nat. 1996;147:287–295. [Google Scholar]
- Qiu M., Yuan Z., Luo H., Ruan D., Wang Z., Wang F., Li Y., Xu R. Impact of pretreatment hematologic profiel on survival of colorectal cancer patients. Tumor Biol. 2010;31:255–260. doi: 10.1007/s13277-010-0024-x. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U. Functional consequences of nutritional anemia during pregnancy and early childhood. In: Ramakrishnan U., editor. Nutritional Anemias. CRC Press; Washington, DC: 2001. pp. 43–68. [Google Scholar]
- Rodrigues S.C., Adornes A.C., dos Santos E.A., Silva R.P., Colares E.P. Surviving probability indicators of landing juvenile Magellanic penguins arriving along the southern Brazilian coast. Braz. Arch. Biol. Technol. 2010;53:419–424. [Google Scholar]
- Rowe A., McMaster K., Emery D., Sangster N. Haemonchus contortus infection in sheep: parasite fecundity correlates with worm size and host lymphocyte counts. Vet. Parasitol. 2008;153:285–293. doi: 10.1016/j.vetpar.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Schalk G., Forbes M.R. Male biases in parasitism of mammals: effects of study type, host age, and parasite taxon. Oikos. 1997;78:67–74. [Google Scholar]
- Selvam R., Baskaran G. Hematological impairments in recurrent Plasmodium vivax infected patients. Jpn. J. Med. Sci. Biol. 1996;49:151–165. doi: 10.7883/yoken1952.49.151. [DOI] [PubMed] [Google Scholar]
- Sinclair A.R. University of Chicago Press; Chicago, IL: 1977. African Buffalo: A Study of Resource Limitations of Populations. [Google Scholar]
- Soulsby E.J.L. Immunology of helminths. Compr. Immunol. 1982;9:487–513. [Google Scholar]
- Stear M.J., Bairden K., Bishop S.C., Gettinby G., McKellar Q.A., Park M., Strain S., Wallace D.S. The processes influencing the distribution of parasitic nematodes among naturally infected lambs. Parasitology. 1998;117:165–171. doi: 10.1017/s0031182098002868. [DOI] [PubMed] [Google Scholar]
- Stevenson L.A., Chilton N.B., Gasser R.B. Differentiation of Haemonchus placei from H. contortus (Nematoda, Trichostrongylidae) by the ribosomal DNA 2nd internal transcribed spacer. Int. J. Parasitol. 1995;25:483–488. doi: 10.1016/0020-7519(94)00156-i. [DOI] [PubMed] [Google Scholar]
- Stien A., Irvine R.J., Ropstad E., Halvorsen O., Langvatn R., Albon S.D. The impact of gastrointestinal nematodes on wild reindeer: experimental and cross-sectional studies. J. Anim. Ecol. 2002;71:937–945. [Google Scholar]
- Stromberg B.E., Gasbarre L.C., Waite A., Bechtol D.T., Brown M.S., Robinson N.A., Olson E.J., Newcomb H. Cooperia punctata: effect on cattle productivity? Vet. Parasitol. 2012;183:284–291. doi: 10.1016/j.vetpar.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Telfer S., Birtles R., Bennett M., Lambin X., Paterson S., Begon M. Parasite interactions in natural populations: insights from longitudinal data. Parasitology. 2008;135:767–781. doi: 10.1017/S0031182008000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer S., Lambin X., Birtles R., Beldomenico P., Burthe S., Paterson S., Begon M. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins D.M., Begon M. Parasites can regulate wildlife populations. Parasitol. Today. 1999;15:311–313. doi: 10.1016/s0169-4758(99)01484-2. [DOI] [PubMed] [Google Scholar]
- Tompkins D.M., Dunn A.M., Smith M.J., Telfer S. Wildlife diseases: from individuals to ecosystems. J. Anim. Ecol. 2010;80:19–38. doi: 10.1111/j.1365-2656.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- Turner W.C., Getz W.M. Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of Etosha National Park. J. Wildl. Dis. 2010;46:1108–1119. doi: 10.7589/0090-3558-46.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyk J.A., Cabaret J., Michel L.M. Morphological identification of nematode larvae of smal ruminants and cattle simplified. Vet. Parasitol. 2004;119:277–306. doi: 10.1016/j.vetpar.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Wilson D.J., Sargison N.D., Scott P.R., Penny C.D. Epidemiology of gastrointestinal nematode parasitism in a commercial sheep flock and its implications for control programmes. Vet. Rec. 2008;162:546–550. doi: 10.1136/vr.162.17.546. [DOI] [PubMed] [Google Scholar]
- Wimmer B., Craig B.H., Pilkington J.G., Pemberton J.M. Non-invasive assessment of parasitic nematode species diversity in wild Soay sheep using molecular markers. Int. J. Parasitol. 2004;34:625–631. doi: 10.1016/j.ijpara.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Yacob H.T., Basazinew B.K., Basu A.K. Experimental concurrent infection of Afar breed goats with Oestrus ovis (L-1) and Haemonchus contortus (L-3): interaction between parasite populations, changes in parasitological and basic haematological parameters. Exp. Parasitol. 2008;120:180–184. doi: 10.1016/j.exppara.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Yochem P.K., Stewart B.S., Mazet J.A.K., Boyce W.M. Hematologic and serum biochemical profile of the Northern elephant seal (Mirounga angustirostris): variation with age, sex, and season. J. Wildl. Dis. 2008;44:911–921. doi: 10.7589/0090-3558-44.4.911. [DOI] [PubMed] [Google Scholar]
- Zarlenga D.S., Higgins J. PCR as a diagnostic and quantitative technique in veterinary parasitology. Vet. Parasitol. 2001;101:215–230. doi: 10.1016/s0304-4017(01)00568-4. [DOI] [PubMed] [Google Scholar]
- Zarlenga D.S., Gasbarre L.C., Boyd P., Leighton E., Lichtenfels J.R. Identification and semi-quantitation of Ostertagia ostertagi eggs by enzymatic amplification of ITS-1 sequences. Vet. Parasitol. 1998;77:245–257. doi: 10.1016/s0304-4017(98)00114-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


