Abstract
Childhood adversity is associated with physiologic dysregulation across multiple biological systems; however, relatively little is known about whether these changes are reversible with intervention. The objective of this review was to examine evidence for the effectiveness of interventions to promote healthy cortisol regulation in children. We selected articles from English-language publications in PubMed and EBSCO databases through 2012. Two independent reviewers assessed articles against eligibility criteria. Eligible studies were randomized controlled or quasi-experimental studies designed to improve relationships, environments, or psychosocial functioning in children and examined cortisol as an outcome. We identified 19 articles. There was substantial heterogeneity across studies with regard to age, selection criteria, intervention design, cortisol assessment, and follow-up duration. Eighteen of the 19 articles reported at least 1 difference in baseline cortisol, diurnal cortisol, or cortisol responsivity between intervention and control participants. Importantly, however, there was remarkable inconsistency with regard to how the interventions influenced cortisol. Therefore, studies that included a low-risk comparison group (n = 8) provided critical insight, and each found some evidence that postintervention cortisol levels in the intervention group approximated the low-risk comparison group and differed from children receiving usual care. In conclusion, existing studies show that cortisol activity can be altered by psychosocial interventions. These findings are promising, not only because they indicate physiologic plasticity that can be leveraged by interventions but also because they suggest it may be possible to repair regulatory systems after childhood adversity, which could inform strategies for reducing health disparities and promoting lasting improvements in health.
Keywords: childhood adversity, toxic stress, cortisol, hypothalamic-pituitary-adrenal axis, infants, children, adolescents, prevention, life course, randomized controlled trial, quasi-experimental study
Animal studies consistently document disruptions in physiologic stress-response system development after adverse early-life experiences,1–3 and human studies also find disruptions in stress-related physiology after exposure to childhood adversity (CA).4–6 Dysregulation in physiologic stress-response systems may be a mechanism through which CA is biologically embedded to influence risk for psychopathology and poor physical health outcomes.4,6,7 Although CA is associated with persistent neurobiological changes across multiple physiologic systems,4,7,8 little is known about whether these changes are reversible with intervention.1,9–11 Over the past 15 years, numerous intervention approaches have been developed to promote healthy stress-response system development in children.1,12 These interventions aim to restore disrupted regulatory systems to prevent mental or physical health problems over the life course.11 To date, these studies have not been reviewed systematically.
Cortisol is one of the most common stress biomarkers within pediatric research. Cortisol is produced by the hypothalamic-pituitary-adrenal (HPA) axis, a physiologic system that governs neuroendocrine responses to stress. Cortisol has a pervasive regulatory influence on multiple bodily systems, including the following: the central nervous system, where it is involved in learning, memory, and emotion; the metabolic system, where it regulates glucose storage and utilization; and the immune system, where it influences the magnitude and duration of inflammatory responses and lymphocyte development.13,14 In healthy individuals, cortisol exhibits a diurnal rhythm characterized by low levels at night, a rise in the hours before waking, a sharp increase 30 to 45 minutes after waking, and a subsequent decline over the rest of the day; cortisol levels increase after social and evaluative stressors.15 CA can have a prolonged influence on cortisol secretion patterns.16–18 Dysregulation can entail both hyper- and hypoactivity, which may be reflected by atypical diurnal secretion and enhanced or reduced responsiveness to stress.13,14,16,19–21 Children exposed to adversity exhibit lower morning cortisol values,22 blunted cortisol awakening response,23 shallow morning to evening slope,21,24–26 greater area under the daytime cortisol curve,24,27 and both blunted28–30 and elevated31,32 cortisol response to acute stressors relative to unexposed children. Dysregulated cortisol secretion has been associated with numerous chronic health problems, including metabolic syndrome,33 coronary artery disease,34 psychiatric disorders,35–37 and others.38–41 Interventions that promote healthy cortisol regulation may therefore be beneficial in preventing a variety of health problems.
The purpose of this review is to systematically examine evidence for the effectiveness of interventions to promote healthy cortisol regulation in children, to make recommendations for future research and prevention-oriented interventions. We focus on cortisol because it is the most commonly used marker in interventions targeting physiologic stress-response systems in children.
Methods
The protocol for this systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (see Supplemental Table 4 for checklist).42
Eligibility Criteria
Eligible studies were randomized controlled trials (RCTs) or quasi experiments, common research designs to estimate the causal impact of an intervention. Quasi experiments share many characteristics with RCTs but they do not entail random assignment to treatment or control conditions.43 To be included, we required that studies were designed to improve social relationships, environments, or psychosocial functioning in children (birth to age 18 years), used cortisol as an outcome measure, and were published in an English-language peer-reviewed journal or book chapter. Studies were eligible regardless of cortisol measurement type (ie, single assessment, diurnal pattern, cortisol responsivity). We excluded studies in which the primary aim was to evaluate the effect of a psychosocial (eg, cognitive-behavioral, mindfulness-based) or medication-based therapeutic treatment of psychiatric disorders on cortisol, to focus on prevention-oriented interventions rather than the considerably more heterogeneous literature on treatments targeting specific mental health impairments.
Data Sources and Search Strategy
The search strategy involved an electronic literature search of all English-language articles published in PubMed and EBSCO databases (PsycInfo, ERIC, CINAHL) from inception through December 2012. Medical Subject Headings (MeSH) of the National Library of Medicine were used to search PubMed, and a similar search was designed for the EBSCO databases (search strategies are provided in Supplemental Table 4). In addition, we searched reference lists of selected articles and relevant review articles for applicable studies.
Study Selection
Studies identified in the database searches were assessed for relevance on the basis of title and abstract by 2 independent reviewers (N.S. and K.A.M.). Relevant articles were obtained in full and assessed for inclusion criteria. Disagreements were resolved through discussion among all authors.
Data Extraction
For each selected article, we extracted data related to study design, sample size, geographic location, sample age at intervention initiation, sample selection criteria (eg, history of foster care or maltreatment; general population), duration of intervention, intervention components, cortisol measurement, cortisol inter- and intraassay variation, length of follow-up after intervention initiation, statistical approach, covariates, and results.
Quality Assessment
All included studies were evaluated for methodologic quality to assess the strength of the evidence provided by each study. We rated 6 components of each study, including design, sample size, cortisol assessment, collection of key covariates, length of follow-up, and cortisol inter- and intraassay coefficients of variation (CVs). We awarded 1 point for each of the following characteristics: RCT design; n ˃100; collection of diurnal cortisol or cortisol responsivity to stress (in contrast to a single-time-point assessment); attention to key covariates to ensure effective randomization, control for confounding, or as exclusion criteria; follow-up of ≥1 year; and inter- and intraassay CVs below the recommended level of 10%.
Data Synthesis
Substantial heterogeneity across studies existed with regard to methodology and populations, which made it impossible to carry out a meta-analysis; therefore, we developed a narrative synthesis of results. We summarized the design characteristics of all studies and described the effectiveness of interventions on cortisol regulation separately by type of cortisol assessment.
Results
Search Results and Description of Included Studies
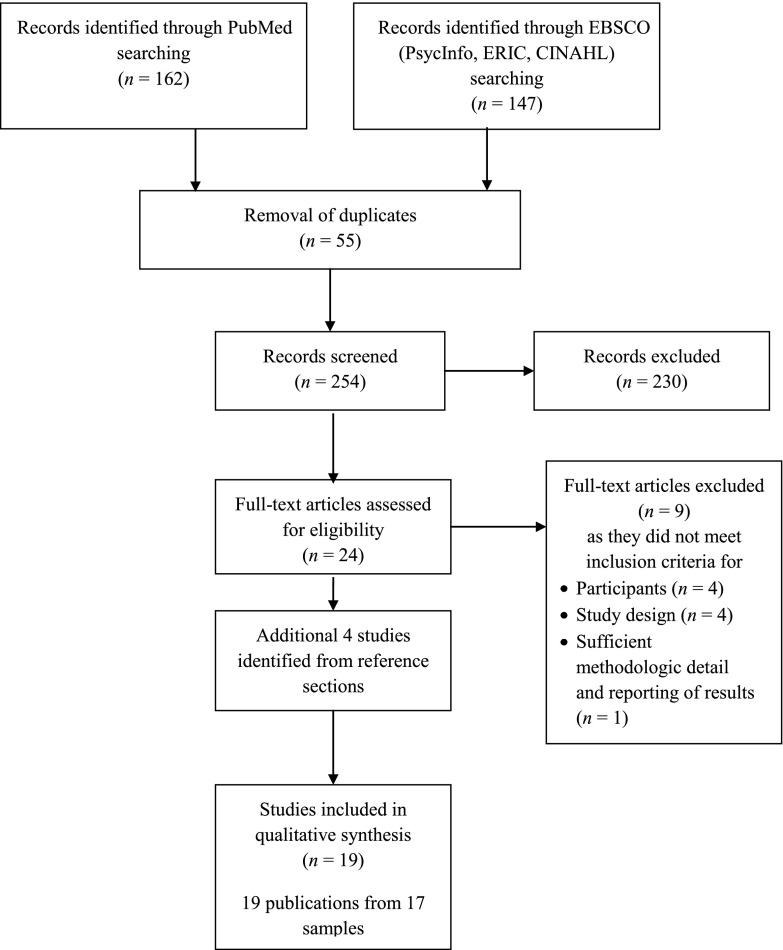
Our search identified 254 titles, and 28 articles were retrieved for closer consideration (see Fig 1). Nineteen articles from 17 studies met inclusion criteria; 1 study resulted in 3 articles44–46; we describe in detail only the initial article44 (Table 1). Study characteristics are summarized in Tables 2 and 3. The first study that met inclusion criteria was published in 1997.47 The majority of studies were RCTs (n = 13)44,45,47–58; quasi experiments were less common (n = 4).59–62 The sample sizes ranged from 1253 to 1197,61 with a median of 126 participants. Twelve studies took place in the United States; the others were carried out in Canada,57 Mexico,61 the Netherlands,52 Romania,47 and Sweden.60 Studies in young children were most common; 7 studies restricted enrollment to children younger than 2 years,47–49,54,57,58,62 whereas 3 studies enrolled children aged 7 or older.53,55,60 Samples were selected on the basis of the following: institutionalization or involvement with foster care (n = 5),44,47,49,50,59 maltreatment history (n = 2),56,62 maternal depression (n = 2),57,58 and other family characteristics associated with developmental risk (eg, parental loss,55 poverty,61 maternal drug use48; n = 6); and 2 studies used samples of children from the general population.53,60 Among the 15 studies with samples identified on the basis of exposure to adversity, 8 studies included a nonexposed comparison group in addition to the control group.44,48–50,56,58,59,62 Intervention duration ranged from <1 month (n = 1)53 to >1 year (n = 6),44,47,54–56,58,61 and it was most common for studies to assess outcomes within ≤6 months after the intervention began (n = 8)48–50,53,57,59,62 or >1 year after intervention initiation (n = 8).28,33,39,41,43,44,46,55 Fifteen studies included intervention components that involved both parents and children, and 2 studies tested interventions that involved only children.53,60 All studies assessed cortisol by using saliva samples. Diurnal cortisol was the most common type of cortisol measure (n = 10), and cortisol reactivity (n = 5) or single cortisol assessments (n = 2) were less common. Two of the 17 studies reported intra- and interassay CVs for cortisol samples above the recommended 10% threshold,44,62 and 5 studies did not report this information.47,48,57,59,60 Only 1 study met all 6 of the study quality criteria,55 although 7 studies fulfilled 5 of the 6 criteria.44,49,50,52,56,58,61
FIGURE 1.
Flow diagram of study selection for the identification of RCTs and quasi-experimental interventions to promote cortisol regulation in children.
TABLE 1.
Summary of Empirical Studies of Preventive Interventions to Promote Improved Cortisol Activity in Infants, Children, or Adolescents
| Number of Studies | Total Studies, % | |
|---|---|---|
| Year of publication | ||
| Before 2005 | 3 | 17.6 |
| 2005–2010 | 8 | 47.1 |
| 2010–2012 | 6 | 35.3 |
| Study design | ||
| RCT | 13 | 76.5 |
| Quasi-experimental | 4 | 23.5 |
| Sample size | ||
| N < 50 | 2 | 11.8 |
| 50 < N < 100 | 6 | 35.3 |
| 100 < N < 200 | 7 | 41.2 |
| N ≥ 200 | 2 | 11.8 |
| Location of study | ||
| United States | 12 | 70.6 |
| Other | 5 | 30.4 |
| Sample age at start of intervention, y | ||
| 0–2 | 7 | 42.1 |
| 3–6 | 3 | 17.6 |
| 0–6 | 4 | 23.5 |
| 7–11 | 1 | 5.9 |
| 12–18 | 2 | 11.8 |
| Childhood risk exposure | ||
| Foster care/institutional care | 5 | 29.4 |
| Maltreatment | 2 | 11.8 |
| Parental death | 1 | 5.9 |
| High-risk families (eg, maternal drug use or depression, low socioeconomic status) | 7 | 41.2 |
| General population | 2 | 11.8 |
| Duration of intervention | ||
| ≤1 month | 1 | 5.9 |
| 1–6 months | 7 | 41.2 |
| 6 months to 1 year | 4 | 23.5 |
| >1 year | 5 | 29.4 |
| Intervention components | ||
| Caregiver- and child-focused | 15 | 88.2 |
| Child-focused | 2 | 11.8 |
| Cortisol measure | ||
| Single assessmenta | 2 | 11.8 |
| Diurnal | 8 | 47.1 |
| Baseline and response | 5 | 29.4 |
| Diurnal and responsivity or single assessment | 2 | 11.8 |
| Attention to key covariatesb | ||
| Yes | 15 | 88.2 |
| No | 2 | 11.8 |
| Cortisol intra- and interassay CVs <10% | ||
| Yes | 10 | 58.8 |
| No | 2 | 11.8 |
| Not reported | 5 | 29.4 |
| Length of follow-up after intervention started | ||
| <6 months | 7 | 41.2 |
| 6–12 months | 3 | 17.6 |
| >1 year | 7 | 41.2 |
N = 17 studies.
Single cortisol assessments may have occurred more than once over time.
Attention to key covariates to ensure that randomization was effective or to determine necessary control covariates or respondents who should be excluded from the sample.
TABLE 2.
Randomized Prevention-Oriented Intervention Studies That Assessed Cortisol Activity in Infants, Children, or Adolescents
| Study | Sample and Child Age at Baseline | Intervention and Follow-up Time | Cortisol Measurea | Results | Scoreb |
|---|---|---|---|---|---|
| Carlson and Earls, 199747 | • Romanian institutions | • Intervention: social and educational enrichment and an improved child-to-caretaker ratio for 13 months | • Diurnal (day 1: 8 am, noon, 7 pm; day 2: 8 am, noon, 5 pm, 6:30 pm, 7 pm) | • Intervention group had lower noontime cortisol compared with controls | 3 |
| • Enrichment program (n = 30) and controls (n = 28); N = 58 | • Follow-up: 6 months after intervention ended | • 2 consecutive days | • Note: differences in slope, AUC, or CAR were not evaluated | ||
| • 2–9 months at baseline | |||||
| Field et al, 199848 | • Polydrug-using mothers (ages 16–21 y) and drug-exposed infants | • Intervention: high school–based 3-month intervention that included educational, vocational, and parenting classes; social and drug rehabilitation; and day care while mothers attended school | • Responsivity | • At 3- and 6-month assessments, intervention group had cortisol levels and change scores that approximated those of the nondrug group, with values for these groups lower than the drug control group | 4 |
| • Intervention and control conditions, and matched non–drug-using controls; N = 126c | • Follow-up 3 and 6 months into intervention | • Before and 20 minutes after mother–infant play interactions | |||
| • Infants enrolled at birth | |||||
| Fisher et al, 200744 | • Foster children entering new placements | • Intervention: enriched foster care included 6–9 months of intensive parent training, 24-hour telephone support, foster parent group meetings, and child-focused treatment | • Diurnal (30 minutes after waking, 30 minutes before bedtime) | • Intervention group exhibited cortisol activity that became comparable to the community comparison children over the study | 5 |
| • Enriched foster care (n = 57), regular foster care (n = 60), and community comparison (n = 60); N = 117 | • Monthly follow-up for 1 year | • 2 consecutive days monthly for 1 year | • Controls exhibited increasingly flattened morning-to-evening cortisol activity | ||
| • 3–6 years at baseline | |||||
| Fisher et al, 201145 | • Subsample from Fisher et al44 who had 1 placement change during first 6 months of intervention and no other change for 6 months | • Intervention and follow-up same as above44 | • Same as above | • Intervention group showed stable and typical diurnal cortisol patterns before and after placement change | 4 |
| • Enriched foster care (n = 36) and regular foster care (n = 35); N = 71 | • Among controls, placement changes predicted blunted morning to evening cortisol decreases | ||||
| Graham et al, 201246 | • Subsample from Fisher et al44 recruited 2 years after study began | • Intervention same as above44 | • Diurnal (30 minutes after waking, 4 pm, 30 minutes before bedtime) | • Enriched-care group was similar to controls in showing a difference in cortisol slope between the week before and the first day of school, but not between the week before and fifth school day | 4 |
| • Enriched foster care (n = 9), regular foster care (n = 7), community controls (n = 21); N = 37 | • Follow-up: 2 years after children enrolled in the intervention | • Collected 1 week before start of school (for 2 consecutive days), first day, and fifth day of school | • Regular care group exhibited a steeper cortisol slope on the fifth day relative to the other 2 groups | ||
| Dozier et al, 200650 | • Children in foster care | • Experimental intervention: 10 weekly in-home sessions to help parents encourage regulatory capacities in their children | • Diurnal (waking and bedtime) | • Experimental intervention and community-based comparison groups had lower cortisol than the educational intervention group for both am and pm cortisol values | 5 |
| • Experimental or educational intervention (n = 60c) and children not in foster care system (n = 104); N = 164 | • Educational intervention: 10 weekly in-home sessions focused on developing language skills | • 2 consecutive days | • No differences between experimental intervention and community comparison groups | ||
| • 2 months to 3 years at baseline | • Follow-up: 1 month postintervention | ||||
| Dozier et al, 200849 | • Children in foster care | • Same interventions as above50 | • Responsivity | • Experimental intervention and community controls had lower cortisol upon arrival compared with children in the educational intervention | 5 |
| • Experimental (n = 46) or educational (n = 47) intervention and children not in the foster care system (n = 48); N = 141 | • Follow-up: 10-week intervention period | • 3 samples: arrival at the laboratory, 15 and 30 min after Strange Situation task | • No group differences in response to the Strange Situation task | ||
| • 13 to 22 months at baseline | |||||
| Brotman et al, 200751 | • Siblings of youth adjudicated for delinquency | • Intervention: 22 weekly sessions and 10 biweekly home visits designed to improve parenting practices and preschoolers’ social competence over a 6- to 8-month period | • Responsivity: arrival at laboratory, before and after social challenge | • Intervention group had increased cortisol levels in anticipation of the social challenge relative to controls | 4 |
| • Intervention (n = 47) and control (n = 45) conditions; N = 92 | • Assessments: baseline and 9 months after intervention began | • Diurnal: 4 samples 1 week after laboratory visit (7 am, 12 pm, 4 pm, 8 pm) | • No intervention effects were observed for postchallenge cortisol or diurnal home assessment (ie, no difference in slope) | ||
| • 33 to 63 months at baseline | |||||
| Bakermans-Kranenburg, 200852 | • Sample from the Netherlands | • Intervention: six 1.5-hour home visits focusing on maternal sensitivity and discipline over 8 months | • Diurnal: 3 samples (waking, before lunch, bedtime) | • Children receiving the intervention with a DRD4 7-repeat allele showed lower cortisol production (AUC) compared with controls | 5 |
| • Families of children with high externalizing behavior | • Follow-up: 1 year after study began | • Intervention was not effective in decreasing cortisol in children without a DRD4 7-repeat allele | |||
| • Intervention (n = 66) and control (n = 64) conditions; N = 130 | |||||
| • 1 to 3 years at enrollment | |||||
| Weigensberg et al, 200953 | • Obese Latino youth | • Intervention: 45-min sessions of Interactive Guided Imagery for 4 consecutive weeks | • Responsivity | • Intervention group exhibited reductions in cortisol for 3 of the 4 sessions; controls did not exhibit reductions | 4 |
| • Intervention (n = 6) and controls (n = 6); N = 12 | • Weekly assessments during intervention | • Before and after each guided imagery session | • In analyses that were averaged across sessions, there was a between-group effect for change in cortisol in the intervention compared with the control group | ||
| • Ages 14–17 years at baseline | |||||
| Bugental et al, 201054 | • High-risk mothers and children (ie, medical problems in children, rural or highly mobile residence, low education) | • Experimental intervention: 1 year of activities to increase knowledge about parenting and access to social and community resources by facilitating problem-solving and information-seeking skills | • Basal | • At years 1 and 3 assessments, children in the experimental program had lower cortisol compared with children in the standard program | 4 |
| • Experimental home visitation program and standard home visitation program; N = 64c | • Standard program: 1 year of education, support, and information on community resources | • Midmorning collection once annually over 3 years | • There was an interaction with time whereby cortisol levels declined for children in the experimental program but not for children in the standard program | ||
| • Mean baseline age: 9 weeks | • Follow-up: 1 and 3 years | ||||
| Luecken et al, 201055 | • Parentally bereaved children | • Intervention: 12-week family-based program to address risk and protective factors associated with adapting to loss of a parent | • Responsivity | • Intervention group had higher cortisol levels across the task compared with controls | 6 |
| • Intervention (n = 78) and controls (n = 61); N = 139 | • Follow-up: 6 years postintervention | • Before and after a conflict discussion task | • No group differences in response pattern | ||
| • Mean age at baseline: 11.5 years | |||||
| Cicchetti et al, 201156 | • Maltreated children (n = 91) | • CPP intervention: attachment-based therapy to encourage sensitive interactions between caregivers and children | • Basal | • CPP and PPI were combined for analysis | 5 |
| • CPP or PPI (n = 56), or controls (n = 35) and community-control children (n = 52); N = 143 | • PPI intervention: education on parenting skills, relaxation techniques, and behaviors that promote social support | • Midmorning collection on 4 occasions over the 2 years | • Initial assessment: no differences | ||
| • Ages 1–3 years at baseline | • Interventions lasted ∼1 year | • Midintervention: intervention group became indistinguishable from the community-control group, and the control group progressively exhibited lower morning cortisol that differed from the intervention and community control children | |||
| • Assessments occurred for 1 year after interventions ended | |||||
| Letourneau et al, 201157 | • Canadian sample | • Intervention: 12 weeks of home-based peer support that included education on maternal-infant interaction | • Diurnal (waking, noon, midafternoon, before bed) | • No intervention effects were observed on cortisol AUC | 3 |
| • Mothers with depression | • Follow-up: 6 and 12 weeks postrandomization | • Collected at baseline and 6 and 12 weeks | |||
| • Intervention (n = 27) and controls (n = 33); N = 60 | |||||
| • Mean baseline age: 5 months | |||||
| Urizar et al, 201158 | • Predominantly Spanish-speaking low-income women at risk of depression during second trimester | • Intervention: 12-week prenatal cognitive-behavioral stress management course on strategies to create a healthy environment for mothers and their infants; booster sessions 1, 3, 6, and 12 months postpartum | • Diurnal (morning and evening) | • At 6 months postpartum, infants whose mothers received usual care had higher average cortisol levels (ie, average of morning and evening values) relative to infants whose mothers received the intervention or infants of mothers in the low-risk control group; no group differences were observed for cortisol slope, am, or pm measures | 5 |
| • Intervention (n = 24), control (n = 33), and a community comparison (n = 29); N = 86 | • Follow-up: 6 and 18 months postpartum | • Collected at 6 and 18 months postbirth | • At 18 months postpartum, no group differences were observed (for am, pm, or average cortisol, or cortisol slope) | ||
| • Infants enrolled at birth |
N = 15 articles. Samples are from the United States unless otherwise stated. CAR, cortisol awakening response; CPP, child–parent psychotherapy; DRD4, dopamine receptor D4; PPI, psychoeducational parenting intervention.
If available, exact times of saliva collection are specified.
Quality score was calculated on the basis of 6 study characteristics: study design, size, cortisol assessment (diurnal or responsivity), key covariates used as controls or exclusion criteria (if necessary), cortisol inter- and intraassay CVs, and length of follow-up from start of intervention.
Sample sizes for each condition not provided.
TABLE 3.
Quasi-Experimental Prevention-Oriented Studies That Assessed Cortisol Activity in Infants, Children, or Adolescents
| Study | Sample Description and Child Age at Baseline | Intervention and Follow-up Time | Cortisol Measure | Results | Scorea |
|---|---|---|---|---|---|
| Fisher et al, 200059 | • Foster children entering new placements | • Intervention: intensive preservice training, and postplacement support and supervision through daily telephone contact, weekly home visits by a consultant, support group meetings, and a 24-hour call line, and children received services from a behavior specialist | • Diurnal: 3 samples (30 minutes after waking, between 10 and 10:30 am, and 30 minutes before bedtime) on 2 consecutive days at baseline and 12 weeks | • For diurnal and single cortisol assessments, trends in data (from baseline to follow-up) suggested cortisol patterns for the intervention group were converging with patterns among the community controls, whereas patterns among the regular foster group were diverging from community controls | 2 |
| • Early intervention foster care (n = 10), regular foster care (n = 10), and a community comparison (n = 10); N = 30 | • Follow-up: 12 weeks after intervention began | • Basal: midmorning cortisol, weekly | |||
| • Ages 4.4–5.4 years at baseline | |||||
| Lindblad et al, 200760 | • Sample from Sweden | • Music intervention: 2 (1-hour) music education classes per week for the academic year (9 months) | • Diurnal (at waking, 30 minutes after waking, 1 hour after lunch, before bed) | • Intervention group had lower afternoon cortisol at the end of the school year; however, no difference in change in cortisol by group over the duration of the study | 1 |
| • Music intervention (n = 16), placebo intervention (n = 17), and no intervention (n = 27); N = 60 | • Control intervention: 1 hour of data education or usual curriculum | • 3 times during academic year | • For the other times of cortisol assessment (ie, waking, 30 minutes after waking, or before bed), there were no intervention effects at the end of the school year or for change over the duration of the study | ||
| • Entering fifth and sixth grades at baseline | • Follow-up: 3 times over 9 months | ||||
| Fernald et al, 200961 | • Sample from rural Mexico | • Intervention: conditional cash transfer program, where cash payments were distributed if children and family members complied with requirements, such as prenatal care, well-baby care, nutrition monitoring, and educational programs | • Basal | • In repeated-measures analyses that included 3 cortisol assessments, the intervention group had lower cortisol relative to controls; change in cortisol in response to the team visit did not differ between groups | 5 |
| • Low-income households | • Follow-up: after 3.5 years of participation | • Responsivity: before and after interview team’s arrival | • Intervention was associated with lower cortisol only among children whose mothers had high depressive symptoms | ||
| • Poverty-alleviation intervention (n = 491) and a comparison group from similar communities (n = 706); N = 1197 | |||||
| • Ages 2–6 years at baseline | |||||
| Bernard et al, 201062 | • Children involved with CPS | • Intervention: placement into foster care | • Diurnal cortisol (waking and bedtime) | • CPS-involved children living with birth parents exhibited a more blunted diurnal pattern (according to slope) relative to children in foster care and differed from low-risk children in bedtime cortisol levels (whereas children in foster care did not) | 3 |
| • Children in foster care (n = 184), CPS-involved children living with birth parents (n = 155), and community comparison from low-risk homes (n = 96); N = 435 | • Average time in foster care was 3.6 months | • 2 consecutive days | |||
| • Ages 2.9–31.4 months at baseline |
N = 4 articles. Samples are from the United States unless otherwise stated. CPS, Child Protective Services.
Quality score was calculated on the basis of 6 study characteristics: study design, size, cortisol assessment (diurnal or responsivity), key covariates used as controls or exclusion criteria (if necessary), cortisol inter- and intraassay CVs, and length of follow-up from start of intervention.
Intervention Effectiveness
Eighteen of the 19 identified articles reported at least 1 significant difference in measures of baseline cortisol, diurnal cortisol, or cortisol responsivity between individuals who received the intervention and controls. For summaries of each article, see Table 2 (RCTs) and Table 3 (quasi experiments). Below, we describe the evidence for intervention effects organized by type of cortisol assessment and presence of a nonexposed comparison group.
Single Cortisol Assessment
Two studies collected cortisol at a single assessment (midmorning in both studies).54,56 One study in infants found that midmorning cortisol levels declined for participants who received an experimental home visitation program but not for participants who received a standard home visitation program.54 In contrast, the other study found that maltreated children who received an intervention and nonmaltreated children had higher midmorning cortisol relative to maltreated children who received standard community services.56
Diurnal Cortisol Assessment
Across the 12 studies that collected diurnal cortisol,44–47,50–52,57–60,62 substantial variation existed in how the data were analyzed. Some studies evaluated differences in slope44–46,50,51,58,59,62; others examined area under the curve (AUC),52,57 individual components of diurnal measures separately (eg, am and pm),44,47,51,58,60,62 or average cortisol across morning and evening measures.58 Some studies used multiple analytic strategies.44,51,58,59,62 Among the 8 studies that examined differences in cortisol slope between intervention and control groups, 3 reported that children in control conditions had blunted morning to evening cortisol decreases relative to children who received interventions.44,45,62 In contrast, in a small pilot study of a foster care intervention, children in the control group had a steeper cortisol slope on the fifth day of a new school year relative to an intervention group and community controls, primarily due to higher morning cortisol levels.46 Four studies did not find a difference in cortisol slope between intervention and control groups50,51,58,59; however, 3 of these studies reported group differences in cortisol at a particular point in the diurnal rhythm (ie, lower am and pm cortisol levels in the intervention group,50 lower averaged am-pm cortisol levels in the intervention group,58 and higher cortisol in the morning, midday, and evening among controls but not among children who received the intervention).59
One of the 2 studies that examined cortisol AUC found that the effect of an intervention for children exhibiting behavior problems on overall daily cortisol production was dependent on genotype, whereby only children with a dopamine receptor D4 7-repeat allele who received the intervention exhibited lower daily cortisol relative to children in the control group.52 In the other study that examined cortisol AUC, no differences between infants of depressed mothers randomly assigned to home-based peer support intervention and matched controls were observed.57 Two studies collected diurnal cortisol and only evaluated individual time points.47,60 A study in institutionalized infants in Romania found that those who received an enriched-care intervention had lower cortisol at midday compared with controls.47 Finally, a study in Swedish children found that students who received a music intervention had lower afternoon cortisol compared with controls; however, there was no difference in change in afternoon cortisol by group from baseline to follow-up.60
Cortisol Reactivity
Three49,55,61 of the 548,49,51,55,61 studies that collected cortisol in the context of a stressor/challenge paradigm found no difference in responsivity between the intervention and control groups. In contrast, infants of polydrug-using mothers who received an intervention had lower cortisol increases after a mother–infant play interaction than the control group, and cortisol reactivity approximated that of a non–drug-using comparison group.48 In another study,51 children who received the intervention had increased cortisol in anticipation of a peer social challenge relative to control children; no differences were observed for cortisol levels after the challenge. In 2 of the studies that did not find differences in cortisol reactivity55,61 intervention effects on overall cortisol levels were observed. Specifically, in a quasi-experimental poverty-alleviation study, children who received the intervention had lower cortisol across 3 assessments (collected within 1 hour) relative to control children.61 In contrast, in a study of a parental bereavement intervention, adolescents who received the intervention had higher cortisol across all measures relative to adolescents in the control group.55 In the third study that found no intervention effect on cortisol reactivity, children in a foster care intervention (and children in the non–foster care comparison group) had lower cortisol values upon arrival at the laboratory compared with controls.49 A final study evaluated cortisol response in the context of a relaxation intervention. Adolescents in a guided-imagery intervention exhibited cortisol reductions for 3 of the 4 relaxation sessions, whereas adolescents in the control group did not exhibit such reductions.53
Studies That Included a Nonintervention Comparison Group
Of the 8 studies44,48–50,56,58,59,62 that included a low-risk comparison group, each found some evidence that the intervention group had posttreatment cortisol values that approximated the low-risk comparison group and differed from the group that received usual care.
Discussion
Key Findings
This systematic review reports on interventions aimed at improving cortisol regulation in children. It reveals growing interest in strategies to promote health by focusing on physiologic regulatory systems, with more than half of the identified articles published in the past 5 years. The reviewed literature supports the notion that cortisol regulation in children can be altered by psychosocial interventions targeting children and their caregivers, with 18 of the 19 articles documenting at least 1 significant difference in cortisol activity. Remarkable inconsistency exists, however, with regard to how the interventions influenced cortisol activity. In light of these diverse response patterns, studies that included a low-risk comparison group provide critical insight into whether interventions result in a pattern of cortisol activity that reflects typical or healthy development. All 8 studies that included a low-risk comparison group44,48–50,56,58,59,62 found some evidence that the intervention group had posttreatment cortisol values that approximated the low-risk comparison group and differed from the control group. This pattern suggests that psychosocial interventions hold promise for promoting healthy regulation of physiologic stress-response systems in children and potentially preventing the onset of health problems later in life. Additional research is needed to identify the specific intervention characteristics and components that have the most beneficial influence on cortisol regulation in children.
Limitations of Previous Research
The findings of this review should be interpreted in light of several limitations of existing studies. First, the number of interventions targeting cortisol regulation is relatively small and little consistency exists across studies in sample age, selection criteria, and intervention designs. Because of this heterogeneity, we were unable to statistically compare intervention effectiveness as a function of sample characteristics or intervention type or duration. For example, only 2 studies44,49 examined an intervention that had previously demonstrated an effect on cortisol activity in children,50,59 whereas the remainder tested unique interventions. Second, analytic approaches varied widely across studies, and a priori rationales for cortisol analysis strategies were rarely provided. For example, some studies compared diurnal cortisol slopes for intervention and control groups, whereas others examined multiple time points separately, averaged levels across the entire day, or AUC. As a result of this variation, findings cannot be readily compared and results must be interpreted with caution given that selective reporting of significant differences is likely. A third limitation relates to inconsistency in the timing of cortisol measures for diurnal sample collection. Cortisol levels change dramatically in the first half hour after waking,63 such that a slope derived from waking to bedtime differs meaningfully from a slope derived from 30 minutes after waking to bedtime. Accordingly, differences in morning sampling time may partially account for inconsistencies in the results of interventions on diurnal cortisol slopes. Fourth, neither effect size estimates nor the necessary information to calculate such estimates were provided in most studies, precluding statistical comparisons of effects across interventions. Finally, existing studies are limited in their collection of long-term follow-up data. Because few had follow-up periods that extended >1 year beyond the conclusion of the intervention, it is unknown whether most interventions had a sustained influence. For example, infants randomly assigned to the intervention had lower cortisol relative to infants receiving usual care at a 6-month follow-up,58 but this effect disappeared at an 18-month assessment. Only 1 study provided insight into how interventions might influence cortisol production into adolescence,55 and no studies followed individuals into adulthood. As this field matures, it will be important to address these limitations.
Recommendations for Future Research
Future intervention studies would benefit from incorporating several methodologic features that have been applied inconsistently in previous investigations. First, RCTs provide the strongest test of intervention effects on cortisol functioning in children exposed to adversity. However, studies that include a nonexposed comparison group provide the most useful information about restoring typical HPA axis regulation. A variety of questions remain unanswered regarding how best to characterize adaptive HPA axis functioning across development.63 Inclusion of a comparison group permits evaluation of whether interventions influence cortisol regulation in ways that approximate patterns in typically developing children. Second, as noted above, incorporation of long-term follow-up assessments would provide critical information about the durability of intervention effects. Third, it is critical for future research to be explicit about a priori comparisons of interest and to avoid comparing single time points or conducting other nonplanned comparisons if results are not significant. Fourth, future studies should report effect sizes to characterize the strength of the intervention to permit comparisons across studies. Fifth, such comparisons would be facilitated by standardized, developmentally specific guidelines for (1) exclusion criteria, (2) covariates to evaluate effectiveness of randomization in RCTs or to adjust for confounding in quasi-experimental designs, and (3) cortisol measurement procedures.
With regard to cortisol measurement, building on previous reviews,15 the measurement of diurnal cortisol levels appears to be the most instructive. We have considerably greater knowledge about which types of diurnal cortisol patterns are typical versus atypical than for other types of cortisol measurements, and diurnal rhythms can be measured comparably across studies more easily than cortisol reactivity, given substantial variation in the types of tasks used to elicit reactivity and the lack of norms and standards denoting what is adaptive in terms of cortisol reactivity.64 At a minimum, measuring diurnal cortisol rhythm requires acquiring a sample in the morning and evening and ideally involves an additional afternoon measure.15 These measurements allow calculation of morning-to-evening slope and within- and between-individual variation across the day using multilevel modeling (when ≥3 measurements are acquired), as well as AUC; the latter 2 measurements are generally considered the gold standard measures of cortisol regulation.65–67 Acquisition of an additional sample 30 to 40 minutes after waking provides the opportunity to examine the cortisol awakening response, another well-established marker of cortisol regulation that can be compared easily across studies.68,69 Recommendations for collecting cortisol specimens in developmental studies, including strategies to maximize compliance with sampling protocols, are provided elsewhere.70
Identifying mediators of intervention effects on cortisol regulation is another important goal for future research. Identifying specific intervention components that produce distinctive effects will allow complex interventions to be distilled into the active ingredients most responsible for improvements, permitting greater ease of replication, dissemination, and improvement in cost-benefit profile. Several studies have explored mechanisms of change with regard to cortisol findings. Fisher and Stoolmiller71 documented reductions in caregiver stress after a caregiver-based intervention for children in foster care, and reductions in perceived stress mediated intervention effects on child cortisol regulation. Another study found that lower levels of engagement in avoidant parenting behaviors mediated the effects of a home visitation program on infant cortisol levels.54 Each of these studies identified specific changes in caregivers that led to improvements in child cortisol regulation, highlighting useful targets for future intervention studies. In addition, although the current review focused on preventive interventions specifically, existing evidence also suggests that psychosocial and medication-based treatments for psychiatric disorders in youth can also improve cortisol regulation.72 Identifying common techniques across both preventive and treatment approaches that result in improved cortisol regulation will provide critical information for future interventions.
Perhaps most important, increasing evidence that adversity disrupts functioning across multiple physiologic systems indicates that future investigations should examine intervention effects on a broader set of regulatory parameters and stress biomarkers.4,73,74 To that end, the concept of allostatic load provides a useful framework for conceptualizing the long-term neurobiological effects of CA. Allostatic load refers to a cumulative toll exerted on multiple systems due to the continued need for physiologic adaptation in response to environmental demands.75 Regulatory systems subsumed within the concept of allostatic load include cardiovascular, metabolic, HPA axis, inflammatory/immune, and autonomic nervous systems.74 Expanding the measured end points of psychosocial intervention studies to evaluate effects across the full range of these regulatory systems will provide valuable information regarding the potential for plasticity after exposure to CA. Biomarkers of functioning in most of these systems exist that are relatively simple and inexpensive to measure, including heart rate and blood pressure (cardiovascular), BMI and waist-to-hip ratio (metabolic), and heart rate variability (autonomic nervous system). Moreover, researchers have recently developed methods to assay inflammatory markers (eg, C-reactive protein76) and viral antibodies (eg, herpes simplex virus-1 antibodies77) from salivary specimens. Although measures of lipids and glucose metabolism currently require blood samples, recent methodologic advances have allowed these markers to be assayed from blood spots, which are less invasive than acquiring venous blood.78 The degree to which psychosocial intervention can improve functioning across multiple regulatory systems is a critical question for future research.
Limitations of This Systematic Review
There are several limitations of the present review to consider. First, studies with null findings may be underrepresented due to publication bias.79 Second, our review does not account for selective reporting of results, which may have biased the evidence toward positive findings.80 Third, the review was limited by less than optimal methodology for many of the included studies (eg, small samples, short follow-up time, high cortisol inter- and intraassay variability, single cortisol assessments). Notwithstanding these limitations, increasing calls for greater attention to the problem of toxic stress in pediatric practice and child advocacy underscore the need for a systematic assessment of existing data on available interventions.8,81 To enhance the utility of this review, we applied a quality-rating score to each article to highlight studies that generated the strongest evidence.
Implications for Policy and Practice
Health promotion and disease prevention are the cornerstones of public health generally and of pediatric practice specifically. Over the past several decades, primary health care for children has made great strides in the prevention of infectious disease (through immunizations), but relatively little progress has been achieved in the reduction in disparities in chronic health impairments that are correlated with socioeconomic disadvantage, child maltreatment, or other adversities associated with toxic stress. In many respects, advances in the latter category require broader, public actions to reduce societal-level precipitants of toxic stress (such as neighborhood violence and endemic substance abuse) that impose significant burdens on families with children. At the individual level, increased understanding of causal mechanisms and the underlying pathophysiology that explains the link between CA and adult disease can inform the development of more effective preventive or early therapeutic interventions targeted toward children at greatest risk.82,83 The studies reviewed for this article reveal that individual- and family-focused social interventions have the potential to positively influence neurobiological functioning, as measured by cortisol regulation. This finding underscores the need for all medical practitioners, and pediatric providers in particular, to consider the use of prevention-oriented interventions for vulnerable children as they become available.12,84 Evaluating the effectiveness of alternative strategies to mitigate the negative effects of toxic stress in children requires valid and reliable measures of physiologic disruptions that are markers of both increased, long-term risk for disease and proximal indicators of intervention impacts that reduce that risk by promoting “physiologic healing.” The achievement of breakthrough outcomes for vulnerable children whose needs are not being met by existing services will thus be enhanced considerably by creative collaborations among stress-biology researchers and intervention scientists.
Conclusions
The studies included in this review reveal that cortisol activity can be altered by individualized, psychosocial intervention. These data are promising, not only because they indicate plasticity in stress-response systems that can be influenced by interventions but also because they suggest it may be possible to repair regulatory systems after exposure to CA. The findings from this review underscore the need to build on current best practices and to design even more effective strategies to both reduce health disparities related to CA and promote lifelong improvements in physical and mental health across all population groups.
Supplementary Material
Glossary
- AUC
area under the curve
- CA
childhood adversity
- CV
coefficient of variation
- HPA
hypothalamic-pituitary-adrenal
- RCT
randomized controlled trial
Footnotes
Dr Slopen conceptualized the systematic review, performed the electronic search, evaluated articles for eligibility, extracted relevant data, interpreted the results, and drafted sections of the initial manuscript; Dr McLaughlin conceptualized the systematic review, evaluated articles for eligibility, extracted relevant data, and drafted sections of the initial manuscript; Dr Shonkoff conceptualized the systematic review, interpreted the results, drafted a section of the manuscript, and critically reviewed the other sections of the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a grant from the Robert Wood Johnson Foundation to fund the Early Childhood Innovation Project, a grant from the W. K. Kellogg Foundation to fund Building Community Partnerships to Address the Roots of Racial and Ethnic Disparities in Health, and a grant from the National Institute of Mental Health to Dr McLaughlin (K01-MH092526). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Gunnar MR, Fisher PA, Early Experience, Stress, and Prevention Network . Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Dev Psychopathol. 2006;18(3):651–677 [PubMed] [Google Scholar]
- 2.Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173 [DOI] [PubMed] [Google Scholar]
- 3.Teicher MH, Tomoda A, Andersen SL. Neurobiological Consequences of Early Stress and Childhood Maltreatment: Are Results from Human and Animal Studies Comparable?. Annals of the New York Academy of Sciences. 2006;1071:313–323 [DOI] [PubMed] [Google Scholar]
- 4.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39 [DOI] [PubMed] [Google Scholar]
- 5.Hunter AL, Minnis H, Wilson P. Altered stress responses in children exposed to early adversity: a systematic review of salivary cortisol studies. Stress. 2011;14(6):614–626 [DOI] [PubMed] [Google Scholar]
- 6.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259 [DOI] [PubMed] [Google Scholar]
- 7.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of Therapeutic Interventions for Foster Children on Behavioral Problems, Caregiver Attachment, and Stress Regulatory Neural Systems. Annals of the New York Academy of Sciences. 2006;1094:215–225 [DOI] [PubMed] [Google Scholar]
- 10.Dozier M, Albus K, Fisher PA, Sepulveda S. Interventions for foster parents: implications for developmental theory. Dev Psychopathol. 2002;14(4):843–860 [DOI] [PubMed] [Google Scholar]
- 11.Bruce J, Gunnar MR, Pears KC, Fisher PA. Early adverse care, stress neurobiology, and prevention science: lessons learned. Prev Sci. 2013;14(3):247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leve LD, Harold GT, Chamberlain P, Landsverk JA, Fisher PA, Vostanis P. Practitioner review: children in foster care—vulnerabilities and evidence-based interventions that promote resilience processes. J Child Psychol Psychiatry. 2012;53(12):1197–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89 [DOI] [PubMed] [Google Scholar]
- 14.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45 [DOI] [PubMed] [Google Scholar]
- 15.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436 [DOI] [PubMed] [Google Scholar]
- 16.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13(3):677–693 [DOI] [PubMed] [Google Scholar]
- 17.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13(3):611–628 [DOI] [PubMed] [Google Scholar]
- 18.Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog Brain Res. 2008;167:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35 [DOI] [PubMed] [Google Scholar]
- 20.Goldman-Mellor S, Hamer M, Steptoe A. Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology. 2012;37(11):1755–1768 [DOI] [PubMed] [Google Scholar]
- 21.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13(3):515–538 [DOI] [PubMed] [Google Scholar]
- 22.Bruce J, McDermott JM, Fisher PA, Fox NA. Using behavioral and electrophysiological measures to assess the effects of a preventive intervention: a preliminary study with preschool-aged foster children. Prev Sci. 2009;10(2):129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Vegt EJM, van der Ende J, Kirschbaum C, Verhulst FC, Tiemeier H. Early neglect and abuse predict diurnal cortisol patterns in adults: a study of international adoptees. Psychoneuroendocrinology. 2009;34(5):660–669 [DOI] [PubMed] [Google Scholar]
- 24.Suglia SF, Staudenmayer J, Cohen S, Wright RJ. Posttraumatic stress symptoms related to community violence and children’s diurnal cortisol response in an urban community-dwelling sample. Int J Behav Med. 2010;17(1):43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Dev. 2010;81(1):252–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dozier M, Manni M, Gordon MK, et al. Foster children’s diurnal production of cortisol: an exploratory study. Child Maltreat. 2006;11(2):189–197 [DOI] [PubMed] [Google Scholar]
- 27.Saridjan NS, Huizink AC, Koetsier JA, et al. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The Generation R Study. Horm Behav. 2010;57(2):247–254 [DOI] [PubMed] [Google Scholar]
- 28.Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interparental conflict and child maladjustment. Dev Psychol. 2007;43(4):918–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacMillan HL, Georgiades K, Duku EK, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biol Psychiatry. 2009;66(1):62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl). 2011;214(1):367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouellet-Morin I, Danese A, Bowes L, et al. A discordant monozygotic twin design shows blunted cortisol reactivity among bullied children. J Am Acad Child Adolesc Psychiatry. 2011;50(6):574–582, e573 [DOI] [PMC free article] [PubMed]
- 32.Smeekens S, Marianne Riksen-Walraven J, van Bakel HJA. Cortisol reactions in five-year-olds to parent-child interaction: the moderating role of ego-resiliency. J Child Psychol Psychiatry. 2007;48(7):649–656 [DOI] [PubMed] [Google Scholar]
- 33.Bjorntorp P, Rosmond R. Hypothalamic origin of the metabolic Syndrome X. In: Hansen BC, Saye J, Wennogle LP, eds. The Metabolic Syndrome X: Convergence of Insulin Resistance, Glucose Intolerance, Hypertension, Obesity, and Dyslipidemias—Searching for the Underlying Defects. New York, NY: New York Academy of Sciences; 1999:297–307 [DOI] [PubMed] [Google Scholar]
- 34.Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Ann Med. 2009;41(3):224–233 [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886(1–2):172–189 [DOI] [PubMed] [Google Scholar]
- 36.Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk. In: DeKloet ER, Vermetten E, eds. Stress Hormones and Post Traumatic Stress Disorder: Basic Studies and Clinical Perspectives. Amsterdam, Netherlands: Elsevier Science BV; 2007:121–135 [Google Scholar]
- 37.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17(5):321–328 [DOI] [PubMed] [Google Scholar]
- 39.Epel ES, McEwen B, Seeman T, et al. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62(5):623–632 [DOI] [PubMed] [Google Scholar]
- 40.Demitrack MA, Dale JK, Straus SE, et al. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73(6):1224–1234 [DOI] [PubMed] [Google Scholar]
- 41.Crofford LJ, Pillemer SR, Kalogeras KT, et al. Hypothalamic-pituitary-adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheum. 1994;37(11):1583–1592 [DOI] [PubMed] [Google Scholar]
- 42.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Boston, MA: Houghton Mifflin; 2002 [Google Scholar]
- 44.Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(8–10):892–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher PA, Van Ryzin MJ, Gunnar MR. Mitigating HPA axis dysregulation associated with placement changes in foster care. Psychoneuroendocrinology. 2011;36(4):531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham AM, Yockelson M, Kim HK, Bruce J, Pears KC, Fisher PA. Effects of maltreatment and early intervention on diurnal cortisol slope across the start of school: a pilot study. Child Abuse Negl. 2012;36(9):666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann N Y Acad Sci. 1997;807(1):419–428 [DOI] [PubMed] [Google Scholar]
- 48.Field TM, Scafidi F, Pickens J, et al. Polydrug-using adolescent mothers and their infants receiving early intervention. Adolescence. 1998;33(129):117–143 [PubMed] [Google Scholar]
- 49.Dozier M, Peloso E, Lewis E, Laurenceau J-P, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Dev Psychopathol. 2008;20(3):845–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dozier M, Peloso E, Lindhiem O, et al. Developing evidence-based interventions for foster children: an example of a randomized clinical trial with infants and toddlers. J Soc Issues. 2006;62(4):767–785 [Google Scholar]
- 51.Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Arch Gen Psychiatry. 2007;64(10):1172–1179 [DOI] [PubMed] [Google Scholar]
- 52.Bakermans-Kranenburg MJ, Van Ijzendoorn MH, Mesman J, Alink LRA, Juffer F. Effects of an attachment-based intervention on daily cortisol moderated by dopamine receptor D4: a randomized control trial on 1- to 3-year-olds screened for externalizing behavior. Dev Psychopathol. 2008;20(3):805–820 [DOI] [PubMed] [Google Scholar]
- 53.Weigensberg MJ, Lane CJ, Winners O, et al. Acute effects of stress-reduction Interactive Guided Imagery(SM) on salivary cortisol in overweight Latino adolescents. J Altern Complement Med. 2009;15(3):297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bugental DB, Schwartz A, Lynch C. Effects of an early family intervention on children's memory: the mediating effects of cortisol levels. Mind Brain Educ. 2010;4(4):156–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luecken LJ, Hagan MJ, Sandler IN, Tein J-Y, Ayers TS, Wolchik SA. Cortisol levels six-years after participation in the Family Bereavement Program. Psychoneuroendocrinology. 2010;35(5):785–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cicchetti D, Rogosch FA, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Dev Psychopathol. 2011;23(special issue 03):789–800 [DOI] [PMC free article] [PubMed]
- 57.Letourneau N, Stewart M, Dennis CL, Hegadoren K, Duffett-Leger L, Watson B. Effect of home-based peer support on maternal-infant interactions among women with postpartum depression: a randomized, controlled trial. Int J Ment Health Nurs. 2011;20(5):345–357 [DOI] [PubMed] [Google Scholar]
- 58.Urizar GG, Jr, Muñoz RF. Impact of a prenatal cognitive-behavioral stress management intervention on salivary cortisol levels in low-income mothers and their infants. Psychoneuroendocrinology. 2011;36(10):1480–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: impact on children’s behavior, neuroendocrine activity, and foster parent functioning. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1356–1364 [DOI] [PubMed] [Google Scholar]
- 60.Lindblad F, Hogmark A, Theorell T. Music intervention for 5th and 6th graders—effects on development and cortisol secretion. Stress Health. 2007;23(1):9–14 [Google Scholar]
- 61.Fernald LCH, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Soc Sci Med. 2009;68(12):2180–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Child Protective Services. Arch Pediatr Adolesc Med. 2010;164(5):438–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adam EK. Emotion-cortisol transactions occur over multiple time scales in development: implications for research on emotion and the development of emotional disorders. Monogr Soc Res Child Dev. 2012;77(2):17–27 [Google Scholar]
- 64.Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30(7):698–714 [DOI] [PubMed] [Google Scholar]
- 66.Fekedulegn DB, Andrew ME, Burchfiel CM, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69(7):651–659 [DOI] [PubMed] [Google Scholar]
- 67.Van Ryzin MJ, Chatham M, Kryzer E, Kertes DA, Gunnar MR. Identifying atypical cortisol patterns in young children: the benefits of group-based trajectory modeling. Psychoneuroendocrinology. 2009;34(1):50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72(1):67–73 [DOI] [PubMed] [Google Scholar]
- 69.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7(1):29–37 [DOI] [PubMed] [Google Scholar]
- 70.Granger DA, Kivlighan KT, Fortunato C, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol Behav. 2007;92(4):583–590 [DOI] [PubMed] [Google Scholar]
- 71.Fisher PA, Stoolmiller M. Intervention effects on foster parent stress: associations with child cortisol levels. Dev Psychopathol. 2008;20(3):1003–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adam EK, Sutton JM, Doane LD, Mineka S. Incorporating hypothalamic-pituitary-adrenal axis measures into preventive interventions for adolescent depression: are we there yet? Dev Psychopathol. 2008;20(3):975–1001 [DOI] [PubMed] [Google Scholar]
- 73.Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Dev Psychol. 2003;39(5):924–933 [DOI] [PubMed] [Google Scholar]
- 74.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci. 2010;1186(1):223–239 [DOI] [PubMed] [Google Scholar]
- 75.McEwen BSE, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101 [PubMed] [Google Scholar]
- 76.Pace TWW, Negi LT, Dodson-Lavelle B, et al. Engagement with Cognitively-Based Compassion Training is associated with reduced salivary C-reactive protein from before to after training in foster care program adolescents. Psychoneuroendocrinology. 2013;38(2):294–299 [DOI] [PubMed] [Google Scholar]
- 77.Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc Natl Acad Sci USA. 2009;106(8):2963–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925 [DOI] [PubMed] [Google Scholar]
- 79.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990;263(10):1385–1389 [PubMed] [Google Scholar]
- 80.Kirkham JJ, Dwan KM, Altman DG, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ. 2010;340:c365. [DOI] [PubMed] [Google Scholar]
- 81.Garner AS, Shonkoff JP, Siegel BS, et al. Committee on Psychosocial Aspects of Child and Family Health. Committee on Early Childhood, Adoption, and Dependent Care. Section on Developmental and Behavioral Pediatrics . Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1). Available at: www.pediatrics.org/cgi/content/full/129/1/e224 [DOI] [PubMed] [Google Scholar]
- 82.Shonkoff JP. Leveraging the biology of adversity to address the roots of disparities in health and development. Proc Natl Acad Sci USA. 2012;109(suppl 2):17302–17307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shonkoff JP, Garner AS, Committee on Psychosocial Aspects of Child and Family Health. Committee on Early Childhood, Adoption, and Dependent Care. Section on Developmental and Behavioral Pediatrics . The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1). Available at: www.pediatrics.org/cgi/content/full/129/1/e232 [DOI] [PubMed] [Google Scholar]
- 84.Cicchetti D. Annual research review: resilient functioning in maltreated children—past, present, and future perspectives. J Child Psychol Psychiatry 2013;54(4):402–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.