Abstract
Many laboratory studies and epidemiological observations confirm that nematodes prevent some immune-mediated diseases. The development of immunologically well-defined laboratory models of intestinal nematode infection has allowed significant advances to be made in understanding the immunological basis of effector mechanisms operating during infection under controlled laboratory conditions. The Heligmosomoides polygyrus- mouse system is used for studies of parasite immunomodulation. H. polygyrus causes a chronic, asymptomatic intestinal infection and effectively maintains both local and systemic tolerance to reduce allergic and autoimmune inflammation. However, exposure of mice to H. polygyrus antigen reduced spontaneous and glucocorticoid-induced apoptosis of CD4- positive T cells in mesenteric lymph node (MLN). In this study we evaluate the proliferation, cytokine secretion, cell cycle progression and expression of apoptosis related genes in MLN CD4 T cells of uninfected and H. polygyrus infected mice ex vivo and in vitro after restimulation with parasite excretory secretory antigen (ESAg), somatic antigen (SAg) and fraction 9 (F9Ag) of somatic antigen. For the first time we explain the influence of H. polygyrus antigens on the intrinsic pathway of apoptosis. We found that the proliferation provoked by fraction 9 and inhibition of apoptosis was dependent on a low Bax/Bcl-2 ratio, dramatical upregulation of survivin, D1 cyclin, P-glycoprotein, and loss of p27Kip1 protein with inhibition of active caspase-3 but not caspase- 8.
Keywords: intestinal nematodes, CD4 T cells, apoptosis, proliferation, cell cycle, survivin, Bcl-2 family protein
Introduction
Gastrointestinal nematodes are long lived and cause chronic infection, probably because of immunosuppression induced during infection. Immunosuppression is obviously beneficial for the nematode and benefits the host because the reduced inflammatory reaction prevents pathology and destruction of the intestine tissue.1 Nematodes suppress the immunity generated by infection and also affect responses to other non-nematode antigens.2 For this reason nematode exposure can prevent or reverse autoimmune diseases but the pathway activated by nematodes to regulate the host’s immune system is unknown.
The Heligmosomoides polygyrus- mouse system is widely used for studies of parasite immunomodulation. H. polygyrus causes a chronic, asymptomatic gastrointestinal infection which reduces eosinophil responses in the airways of asthmatic mice3; reduces established colitis through the opioid pathway4 and causes EAE remission5,6. During infection, fragments of antigen are presented by antigen presenting cells (APC) to T cells locally and after migration of the APC, in mesenteric lymph nodes (MLN). In the chronic phase of infection, immunosuppression and the low level of cytokines produced by T cells of MLN did not result from programmed cell death and the high survival of MLN lymphocytes with the CD4 phenotype; CD4+CD25- and CD4+CD25hi were detected. The inhibited apoptosis of CD4- positive but no other T cells in mice infected with the nematode was connected with the apoptosis inhibitor Bcl-2 protein7 and FLICE-like inhibitory protein (FLIP) overexpression which are transcriptionally regulated by the nuclear factor kappa B (NFkB). The most active fraction in the induction of proliferation, inhibition of apoptosis and activation of NFkB in CD4+ T cells was fraction 9 of somatic antigen of adult worms.8 The cause of this resistance of CD4+ T lymphocytes to apoptosis in H. polygyrus infection is not fully understood.
In this study to explore the mechanism by which CD4+ T cells are resistant to apoptosis, we analyzed proliferation, cytokine secretion, cell cycle alterations and expression of apoptosis related proteins in pure MLN CD4+ T cells of uninfected and H. polygyrus infected mice ex vivo and in vitro after restimulation with parasite excretory secretory antigen (ESAg), somatic antigen (SAg) and fraction 9 (F9Ag).
For the first time we explain the mechanism by which H. polygyrus antigens inhibit apoptosis. We show that increased CD4+ T cell proliferation is provoked by fraction 9 and inhibition of apoptosis and increase in G2/M cell cycle phase is dependent on low Bax/Bcl-2 ratio, dramatic overexpression of survivin, D1 cyclin, P-glycoprotein (Pgp) and loss of p27Kip1 protein. The inhibition of apoptosis is caspase-3 dependent but independent of caspase-8.
Results
H. polygyrus increased the proliferation of total MLN T cells
To detect the effect of H. polygyrus on long-term proliferation, MLN cells of control and infected mice were seeded on 96-well plates and treated with the previously determined concentration of antigens and CD3/CD28 antibody for 48h−264h and then analyzed by MTS assay (Fig. 1). The cells of infected mice proliferated longer than cells of control mice. The trypan blue exclusion assay (data not show) confirmed the survival in MLN cells as a consequence of H. polygyrus infection and antigen treatment. MLN cells of control mice proliferated intensively after stimulation of TCR and CD28 receptors but not after nematode antigen. MLN of infected mice proliferated weakly after nonspecific stimulation of TCR and CD28 receptors, ESAg and SAg but the F9Ag induced strong and long lasting proliferation of the cells.
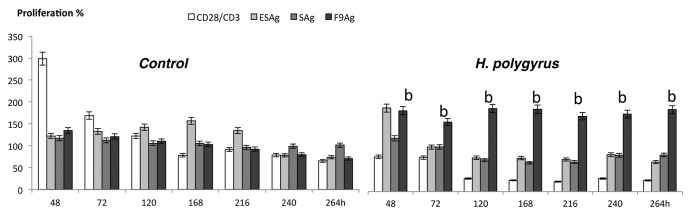
Figure 1. MLN cell proliferation after stimulation with total H. polygyrus ES (ESAg) and S antigen (SAg) and fraction 9 (F9). The antigen effect on stimulation of MLN cell proliferation was calculated using the formula: Proliferation % = (ODAg/ODM) × 100. Where (ODAg) indicates the optical density of the tested antigen and (ODM) indicates the optical density of the control sample with medium alone. Cell proliferation was assayed daily. The experiments were done in triplicate. Bars represent the mean ± SE of six mice of a representative experiment (n = 6). Statistical significance between groups (control and infected) was assessed by ANOVA. ap < 0,05 compared with untreated cells (MEDIUM) within the same group; bp < 0,05 compared with cells with other group treated by the same manner.
Proliferative response to H. polygyrus of CD4+ T cells
CFSE-labeled purified CD4+ T cells were cultured with or without stimulants for seven days (Fig. 2). CD4+ T cells from control mice showed the mean percent of proliferated CD4+ cells and the antigens did not influence the proliferation significantly. The CD4+ T cells of infected mice proliferated more and faster than cells of control mice. CD4+ T cells proliferated to 6 generation in response to stimulation with fraction 9.
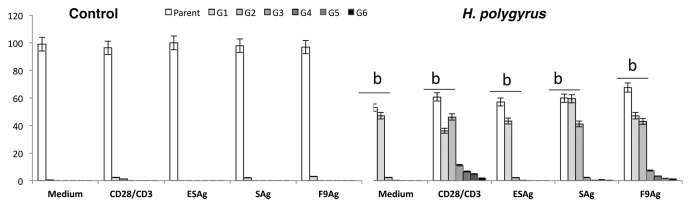
Figure 2. Mean percentage of CD4 T cells in different divisions of proliferation process in control uninfected mice and mice infected with H. polygyrus. MLN cells were stained with CFSE and were adjusted to 2 × 105 cells ⁄well in 96-well plates Cells were in medium alone or were stimulated with CD28/CD3 antibody and H. polygyrus antigens in a final volume of 200 μL. The plates were incubated at 37 °C with 5% CO2 for 7 d. The cells were harvested and stained with anti-CD4 mAb. Flow cytometry FACS data analysis was performed using II Software (B). The numerical values for proportions of proliferated cells at each cell generation were used for statistical analysis. Data from individuals were pooled and the mean percent of CD4+ T cells in each division was presented (A). Statistical significance between groups (control and infected) was assessed by ANOVA. ap < 0.05 compared with untreated cells (MEDIUM) within the same group; bp < 0.05.compared with cells with other group treated by the same manner. Histogram shows unstimulated CFSE stained CD4 T cells of control mice (a) and infected with nematodes (b).
Cytokine concentration in MLN cell culture
We measured the concentrations of IL-2, IL-12, IL-6, IL-22, IL-17A, IL-10, and TGF-β in MLN cell cultures of both control and infected mice (Fig. 3). Nematode infection drastically enhanced the concentration of IL-22 and IL-10 and partly enhanced IL-17A, TGF-β, IL-2 and IL-12. Fraction 9 suppressed regulatory cytokines IL-10and TGF-β synthesis and enhanced IL-22 and IL-17A production.
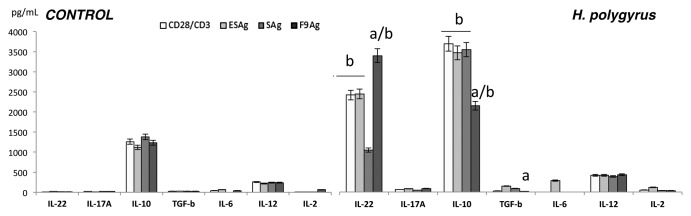
Figure 3. Concentration of IL-2, IL-6, IL-22, IL-17A, IL-10, IL-12 and TGF-β in cultures of MLN cells from uninfected and day 15 infected with H. polygyrus mice. Cells (5 × 105 ⁄ well) were treated with excretory–secretory (ESAg), somatic antigen (SAg) and fraction 9 (F9) and cultured for 7 d. Concentrations of cytokines were measured by specific ELISA. A representative result from three independent experiments is shown. Bars represent the mean ± SE of five mice of representative experiment (n = 5). Statistical significance between groups (control and infected) was assessed by Anova. ap < 0.05 compared with untreated cells (MEDIUM) within the same group; bp < 0.05 compared with cells with other group treated by the same manner.
H. polygyrus infection effects on the pure CD4 T-cell-cycle
H. polygyrus infection resulted in a decrease in the G0/G1 phase and an increase in G2/M cell cycle phase compared with CD4+ T cells of control untreated cells (Fig. 4).
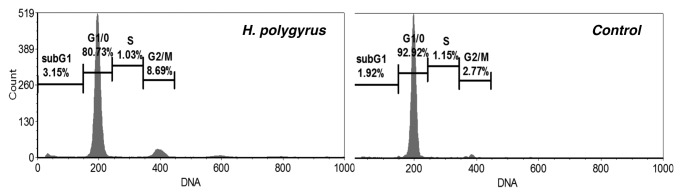
Figure 4. Effects of H. polygyrus infection on CD4 T cell-cycle progression. CD4+ T cells were negatively selected by magnetic field-assisted cell sorting. FACS profiles of cells from control mice (A) and infected mice (B) stained with PI for DNA content are shown. A cell cycle-DNA content analysis was performed. The number on each panel shows the percentage of hypodiploid nuclei. 20000 CD4+ T cells were analyzed for PI fluorescence on a Becton Dickinson FACScalibur.
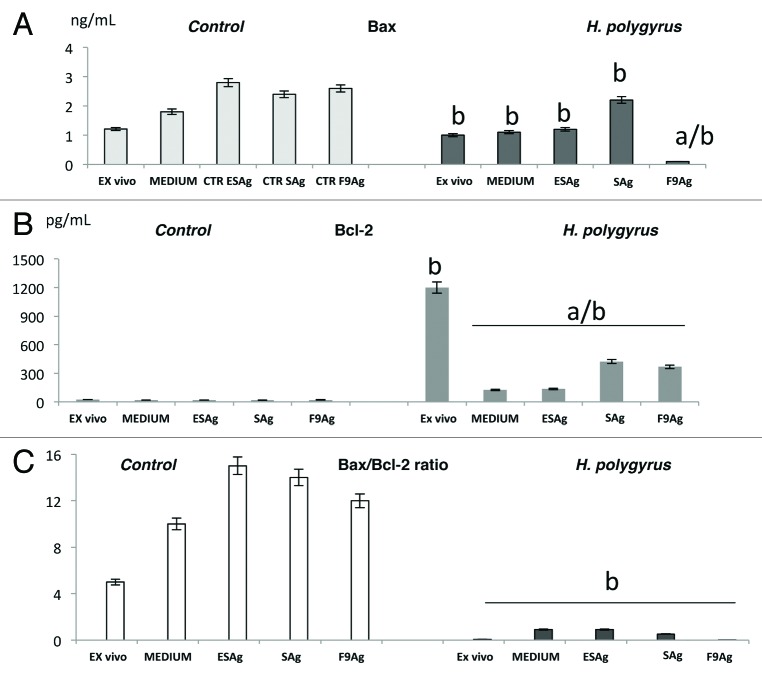
Effects of infection on Bax and Bcl-2 protein expression in CD4+ T cells
Bax and Bcl-2 protein levels in the pure CD4+ T cells were measured by enzyme-linked immunosorbent assay after cell permabilization. H. polygyrus infection increased the expression of Bcl-2 protein and inhibited the concentration of Bax protein observed ex vivo. Nematode antigens exposure led to slow increases in Bax expression and inhibition of Bcl-2 protein expression (Fig. 5A and B). The ratio of Bax to Bcl-2 protein determines the susceptibility of cells to apoptosis signals; therefore the Bax/Bcl2 ratio was calculated in infected and uninfected cells (Fig. 5C). The ratio of Bax/Bcl-2 in the infected cells ex vivo and in vitro especially after F9Ag treatment was dramatically lower than in the untreated cells.
Figure 5. Bcl-2 and Bax expression in CD4 T cells ex vivo and in vitro after antigens treatment and in medium alone of control mice and mice infected with H. polygyrus(A and B). CD4+ T cells were negatively selected by magnetic field- assisted cell sorting directly from MLN or from in vitro cultures. The Bax to Bcl-2 ratio was calculated as mean ± 2SE following the formula: Bax/Bcl × 100% (C). Statistical significance between groups (control and infected) was assessed by ANOVA. ap < 0.05 compared with untreated cells (MEDIUM) within the same group; bp < 0.05 compared with cells with the other group treated in the same manner.
Effects of H. polygyrus on active caspase-3 and caspase-8 protein expression in CD4+ T cells
There was no inhibition of expression of active caspase-8 between the cells of infected and uninfected cells cultured alone and restimulated with ES and S antigens (Fig. 6). However, H. polygyrus infection and restimulation with the antigens inhibited caspase-3 activity. Interestingly, the immunoreactivity of caspase-3 and -8 was dramatically upregulated in CD4+ T cells of control mice in the presence of F9 with significant downregulation of caspase-3 but not caspase-8 in the cells of infected mice.
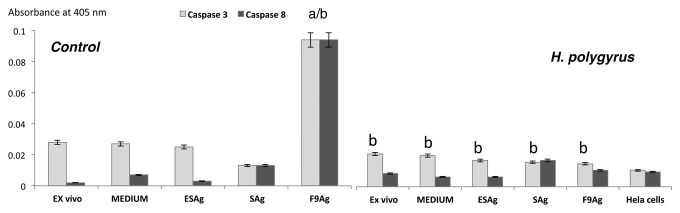
Figure 6. Active caspase-3 and -8 protein expression in CD4 T cells ex vivo and in vitro after antigen treatment of control and infected with H. polygyrus mice. CD4+ T cells were negatively selected by magnetic field-assisted cell sorting directly from MLN or in vitro cultures restimulated with H. polygyrus total ES, S antigen and fraction 9 or cultured alone at 37 °C with 5% CO2 for 7 d. Results are presented as the means of the three replicates from three independent experiments. Statistical significance between groups (control and infected) was assessed by ANOVA. ap < 0.05 compared with untreated cells (MEDIUM) within the same group; bp < 0.05 compared with cells in the other group treated in the same manner.
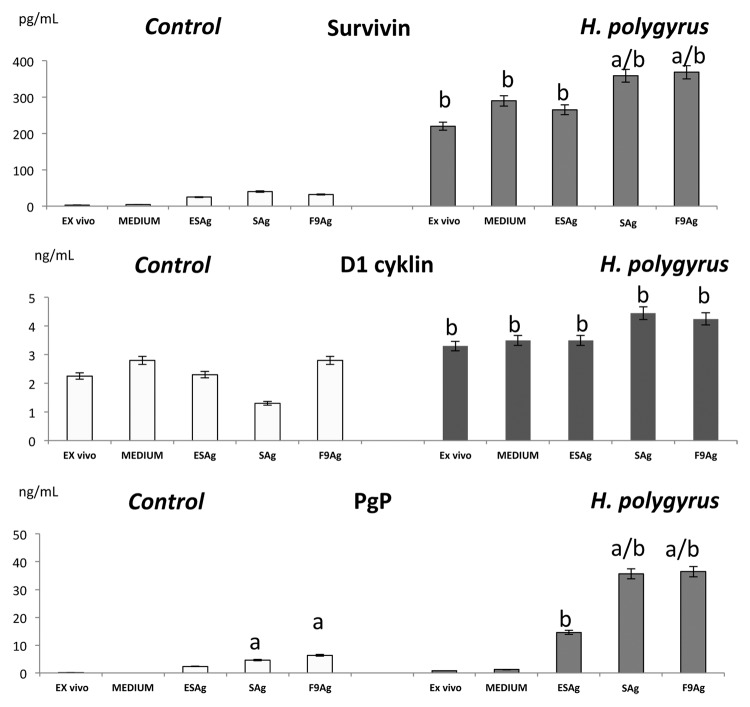
Apoptosis- related genes expression in CD4+ T cells
Expression of apoptosis and cell cycle related proteins was estimated by the protein level in the enzyme-linked immunosorbent assay (Fig. 7) and confirmed by real time quantitative PCR (qRT-PCR, Fig. 8). In the control cells, mRNA expression of survivin was not detected and a very low level was detected in the immunoreactivity assay. The infection with H. polygyrus caused a significant increase in survivin expression that was confirmed at the protein level. Stimulation CD4+ T cells with somatic antigen and fraction 9 increased the expression of survivin.
Figure 7. Effect of H. polygyrus infection on survivin, cyclin D1 and PgP concentration in CD4T cells. For the protein detection, CD4+ T cells were permabilized and Cyclin D1, permeability protein Pgp and survivin levels were measured by an enzyme-linked Immunosorbent Assay. Data are representative for one of three biologically independent experiments. Statistical significance between groups (control and infected) was assessed by ANOVA. ap < 0.05 compared with untreated cells (MEDIUM) within the same group; bp < 0.05 compared with cells in the other group treated in the same manner.
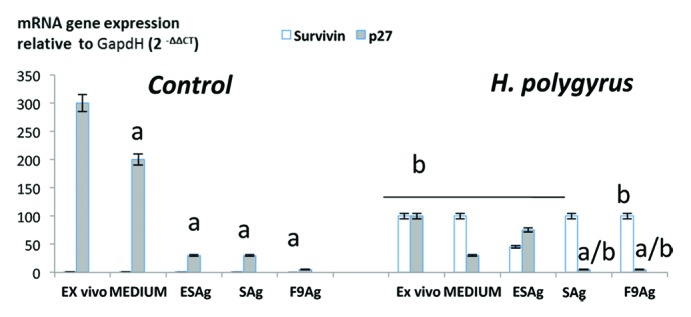
Figure 8. Expression of apoptosis- related proteins; survivin, cyclin dependent kinase inhibitor p27Kip1 by pure CD4 lymphocytes of control mice and infected with H. polygyrus mice. Ct data for p27 Kip1 protein and survivin have been normalized to the housekeeping genes GAPDH and corrected for the total control mean value using Light Cycler Nano Software-1.0. The mean fold change and standard error of target gene expression were calculated from three independent biological replicates. Statistical significance between groups (control and infected) was assessed by ANOVA. ap < 0,05 compared with untreated cells (MEDIUM) within the same group; bp < 0,05 compared with cells with other group treated by the same manner.
At the transcription level there were significant differences between the expression of the cyclin dependent kinase inhibitor p27Kip1 in the cells of nematode infected and control mice. The proliferation of CD4+ T cells was correlated with increased expression of D1 cyclin and with a downregulation of the cell cycle inhibitor p27Kip1. Expression of p27Kip1was drastically downregulated in CD4+ T cells of mice infected with nematodes (ex vivo) and after re-stimulation with antigens but the effect was not induced by fraction 9.
Pgp, the product of the multidrug resistance 1 MDR1 gene was detected in CD4+ cells of control mice at a very low level, However nematode infection lead to the upregulation of Pgp expression especially by SAg and fraction 9.
Discussion
Normally, T cells proliferate in response to stimulation with parasite- derived antigens and proinflammatory cytokines, and activated T cells undergo programmed cell death after the infectious agent has been cleared. In our previous studies H. polygyrus antigen inhibited CD4 positive T-cell sensitivity to spontaneous and glucocorticoid- induced apoptosis by upregulation of Bcl-2 protein.7,8 To demonstrate the mechanism of CD4 T-cell resistance to apoptosis the proliferation, cell cycle alterations and expression of several apoptosis related genes was investigated in cells of normal and H. polygyrus infected mice ex vivo and in vitro.
It was postulated that restimulation in vitro of already activated T cells potently induces Fas/FAsL expression and causes autocrine or paracrine cell death.9 However, H. polygyrus antigen did not induce apoptosis by this way. Interestingly MLN cells of infected mice proliferated weakly after nonspecific stimulation of TCR and CD28 receptors and after stimulation with ESAg and SAg than cells of control mice in the long lasting proliferation experiments. However F9 induced strong and long lasting proliferation of MLN cells and the number of CD4 T cell generations was rising to six generation during seven days of incubation. T lymphocytes divide once every 15−20h10 and the proliferation level of CD4 T cells is dependent on the cytokine milieu. However H. polygyrus infection caused negative effect on the γc chain signaled IL-2 and IL-6 and IL-12 which promote survival of activated T cells ex vivo and in vitro by increased expression of Bcl-2 protein. Fraction 9 promoted higher production of important pro-inflammatory cytokines; IL-22 and IL-17A by T cells and inhibition of regulatory IL-10 and TGF-β1 production as opposed to ES and somatic antigens inducing TGF-β and IL-10 production by MLN T cells. TGF-β and IL-10 cytokines have the potential to downregulate the immune response during nematode infection.11
According to the proliferation results we have shown the shortening of G1/0 phase of cell cycle and increased G2/M phase of the CD4+ T cells of mice infected with H. polygyrus compared with the cells of control mice. Shortening of the G1 phase resulting in quicker cell cycle is probably associated with increased expression of D1 cyclin. In purified CD4 lymphocytes ex vivo and in the stimulated cells with AgS and F9 especially D1 expression was increased. This cell cycle progression correlated with a downregulation of the cell cycle inhibitor Cyclin Dependent Kinase Inhibitor p27Kip1. It is suggested that cell surviving in G2/M phase is specifically connected with survivin expression12 and is associated with microtubules of the spindle apparatus at the beginning of mitosis.13 Cells are most sensitive to survivin targeting in mitosis.14 In CD4+ T cells of infected mice survivin was expressed at high levels. Apoptosis can be induced at any point during the cell cycle but sensitivity to apoptosis varies greatly at different points in the cell cycle. In many long lived transformed cells apoptosis and cell cycle arrest is induced exactly at G2/M.12
Apoptosis is a network of signals which act through two major apoptotic pathways: the extrinsic cytokine dependent death receptor pathway via caspase-8 and the intrinsic mitochondrial pathway via caspase-9 which shifts the balance in the Bcl-2 family proteins toward the proapoptotic members and consequently toward caspase-3 meditated apoptosis.13 Among the various factors responsible for regulation of T-cell apoptosis, the families of Bcl-2 related proteins play a pivotal role15 and the BCL-2 gene is associated with leukemia and lymphoma. Sensitivity or resistance to cell survival is not absolute but reflects the relative balance between the concentration of antagonist Bcl-2 and agonist Bcl-x. In the current study, H. polygyrus infection and restimulation with nematode antigens inhibited the intrinsic pathway of apoptosis. The presented results demonstrated the predominance of Bcl-2 protein over Bax protein and the Bax/Bcl-2 ratio in CD4 T cells of infected mice especially after restimulation with fraction 9 was very low.
The resistance to apoptosis of CD4 T cells of infected mice via the intrinsic pathway may be explained by the Bax/Bcl-2 ratio and expression of active caspases. Caspase-8 is an initiator caspase but caspase-3 leads to dramatic morphologic changes on apoptosis. H. polygyrus infection and somatic antigens downregulated the expression of caspase-3 and slightly upregulated caspase-8. The activation of caspase-8 could be provoked by TNFα. The activation of caspase-8 is strictly related to TNFα concentration and high level of TNFα is detected in the MLN cells of mice infected with H. polygyrus (Doligalska et al., 2006). However the apoptosis through TNFα signaling is induced by cdk-inbitor p27Kip1 (Jaruga-Killeen et al., 2005) and during infection expression of the protein was inhibited. It was recently suggested that caspase-8 activity at low concentration induced T cell proliferation after TCR stimulation and this may explain why H. polygyrus infection does not induce apoptosis through caspase 8 activity.16
The dramatically high expression of survivin in the CD4 T cells of infected mice and very low level of them in control cells of the same organs, fully confirmed inhibition of apoptosis on the intrinsic caspase independent pathway. The recent data are confused to the general mechanism by which survivin protects cells from apoptosis. However our results confirmed that survivin activates caspase-314 but not caspase-8,17 and survival of CD4 T cells appear to occur independently of procaspase-8 processing. Interestingly survivin was not detected in the resting peripheral blood lymphocytes but only in T-cell leukemia lines and in developing T cells in thymus.18 Expression of survivin is regulated by NFκΒ activation.19 Upon stimulus by H. polygyrus antigens, NFκΒ (p50) is released and translocated to the nucleus where it regulates gene transcription.8 NFκB promotes cell survival and cell cycle progression by regulating the expression of several genes involved in the cell cycle machinery such as cyclins, Bcl-2 family protein and survivin.20 It has been suggested that NFκΒ may induce the expression of the multidrug resistance P-glycoprotein in lymphocytes20 and in consequence Pgp confers long-term resistance to caspase-dependent apoptotic stimuli in leukemia T cells and normal cells.21 Pgp upregulation observed in CD4 T cells of mice infected with H. polygyrus ex vivo and after antigen restimulation might be involved in the inhibition of caspase dependent apoptosis.
Taken together, during H. polygyrus infection inhibition of apoptosis and cytokines production, increase in G2/M cell cycle phase is dependent on low Bax/Bcl-2 ratio, dramatic overexpression of survivin, D1 cyclin, P-glycoprotein (Pgp), loss of p27Kip1 protein and inhibition active caspase-3 but not caspase- 8 indicates the inhibition of intrinsic pathway of apoptosis in MLN CD4 T cells. Fraction 9 H. polygyrus of somatic antigen inhibits apoptosis and provoke long-term proliferation of these cells. The general effects observed in CD4 T cells of infected with H. polygyrus mice and treated with antigens in vitro seem to be interconnected as a consequence of antigenic composition which may variably effect on the T-cell survival. However fraction 9 which is connected with nematodes female reproductive system (manuscript in preparation) and contain proteins such as Hsp-60, calumenin, ferritin, galectin and thrombospodin which increase survival of T cells8 might keep the cells in the cell cycle during the infection and increase the frequency of acquired T-cell somatic mutation.
There are limited evidence for an association between intestinal nematode infection and T-cell leukemia. Asymptomatic carriers of human T-cell leukemia virus HTLV-1 developed clinical leukemia faster if they had a concurrent infection with Strongyloides stercoralis, possibly through stimulation of the proliferation of HTLV-1 infected cells.22 However, there is no evidence that chronic infection with the intestinal nematode could be carcinogenic for mesenteric lymph node lymphocytes.
Undoubtedly, treatment with living nematodes gives the best and fastest inhibition of autoimmune diseases.23 However, host parasite relationships are complex and there may be several mechanisms by which parasites could influence the survival of host cells and the therapeutic potential of intestinal nematodes needs to be closely examined for potential adverse side effects.
In immunocompromised hosts or in patients receiving immunosuppressive therapy with whole nematode larvae, nematode pro-oncogenic molecules could be a cofactor of T-cell leukemia and infection could increase the risk of malignant transformation. Cancer is a multifactorial disease and the proposed therapy with nematode antigens could have an important role in this disease control.
Materials and Methods
Mice
Pathogen-free male BALB/c mice were infected orally at eight weeks of age with 200 larvae (L3) of H. polygyrus. Mice were divided into two experimental groups: one experimental group consisted of six mice infected for 15 d and the second group consisted of uninfected, control mice. Animals were sampled individually. Mice were killed under carbon dioxide anesthesia. All experiments were performed in triplicate to ensure accurate results. Results of only one representative experiment are shown. The experiment was conducted in accordance with the guidelines of the Local Ethical Committee.
Antigens of adult worms
Adult worms were collected from BALB/c mouse intestine 15 d post infection using the Baermann technique.24 500 adult nematodes were lysed on ice in 0.5 mL of PBS using an ultrasonic device. The samples were then centrifuged 18 000 g, 5 min, 4 °C, the supernatant (somatic antigen, SAg) was sterile-filtered using a 0.2 μm syringe filter (Milipore, UK). One hundred worms in 0.5 ml of RPMI medium supplemented with penicillin (100 U/ml), streptomycin (100 mg/ml), and L-glutamine (2 mM, Gibco) were cultured aseptically at 37 °C under a humidified atmosphere of 95% air and 5% CO2. The secretory products produced by worms for the first 24 h which contains about 20 endotoxin units/mg protein (LAL test, SterBios) were rejected. After the next 14 d, supernatants containing excretory/secretory products (ESAg) of the nematode protein (<5 endotoxin units/mg) were collected and sterile filtered using a 0.20 μm filter (Sarstedt). Protein concentration was measured by the Bradford technique.25 The antigens were stored at −80 °C until used.
Separation of antigenic fractions
Separation of somatic antigen fractions was performed using high-pressure liquid chromatography (HPLC, Integrity coupled with mass detector, Waters) on ProteinPak column (Waters); 100 μL of antigen solution was loaded onto the column and eluted isocratically with PBS (pH 7.4) at a flow rate of 0.4 mL per minute and fractions of 0.5 mL were collected starting when protein presence was detected at λ = 280 nm. The protein concentration in each fraction was estimated. The fractions were stored at −80 °C until use.
Cell preparation
The mesenteric lymph nodes (MLN) were isolated aseptically from control and infected mice and pressed through a nylon cell strainer (BD, Falcon) to produce a single cell suspension. MLN cells were washed and re-suspended in complete medium- RPMI 1640 (Gibco) supplemented with 5% heat inactivated fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (100 μg/ml), L-glutamine (2 mM), and β-mercaptoethanol (1 U/ml) (Gibco). Cell viability, as determined by trypan blue exclusion, was greater than 96%.
The effect of dose of antigens and fractions on cell apoptosis and proliferation was determined in a preliminary study7,8. To examine the pro-proliferative and anti-apoptotic effects of antigens on the CD4+ T-cell subpopulation, the cells were treated with different dose of antigen; 0,5, 1, 5, 10, 20, 50, and 100 μg for 48−168h. A maximal effect of Ag on CD4+ T cell in vitro (the lowest level of apoptosis and the highest level of proliferation) was found with a dose of 5 μg at 120h. Apoptosis was measured by ssDNA apoptosis test (data not shown). The assay was performed following the manufacturer's protocol using an ssDNA Apoptosis ELISA Kit (Chemicon International, Inc.).
Long-term proliferation for MTS assay
Total MLN cell proliferation was estimated by using the MTS colorimetric method (the Cell Titer 96® Aqueous One Solution Cell Proliferation Assay, Promega). MTS (3-[4,5- dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2[4-sulfophenyl]- 2H-tetrazolium) is reduced by dehydrogenase enzymes in metabolically active cells producing soluble colored formazan in tissue culture medium. Tetrazolium salt reduction is good indicator of cell growth and has been used in models for screening cytocidal chemical agents or cell growth promoting factors and cytokines. The quantity of formazan products, measured at 490 nm absorbance after 4h incubation time, is directly proportional to the number of living cells in the culture.
Cultures were incubated in humidified 5% CO2 atmosphere for 336 h (14 d) at 37 °C. After 48, 72, 120, 168, 216, 240, 264 h with 5 μg/ml of ESAg, SAg or F9Ag or with medium alone at total volume of 200 μl. The cells were stimulated with antigen only one time. During the last 4 h of the culture, 20 μl of MTS solution were added to each well and incubated under the same conditions. The absorbance was measured at 490 nm in a spectrophotometer (u-Quant, BD, Costar). Each experiment was repeated for three times with four wells per dilution in each run.
The antigen effect to stimulate PBMC proliferation was calculated using the following formula: Proliferation % = (ODAg/ODM) × 100. Where (ODAg) indicates the optical density of the tested antigen and (ODM) indicates the optical density of the control sample with Medium alone.
Proliferation measurements
MLN cells were stained with 5 mM stock of the stain 5,6-carboxyfluroescein diacetate succinimidyl ester (CFSE, Invitrogen, Molecular Probes). Freshly isolated cells were incubated for 15 min at RT in the dark with 5 μM CFSE in PBS/0,1% BSA. After a total of three washes with RPMI 1640, the cells were re-suspended in fresh medium and cultured.at a concentration of 2 × 105 cells per well in 96-well flat-bottom plates (Costar). Cells were incubated with 5 μg/mL of complete somatic antigen (AgS), 5 μg/mL of excretory-secretory antigen (ESAg), of 5 μg/mL antigenic fractions or costimulated with anti-CD3/CD28 antibodies; 0.5 μg/ml anti-CD3 (plate bound) and 0.5 μg/mL anti-CD28 antibodies (BD Biosciences, PharMingen) or with complete medium alone. Cells were harvested at day 7 of CFSE culture and washed three times with PBS (pH 7.2). The harvested cells were stained with anti-CD4-PerCP antibodies (BD Biosciences, PharMingen). The cells were examined on a BD FACS Calibur Flow Cytometer. A minimum of 20 000 events were acquired for each sample. Flow cytometry (FACS) data analysis was performed using II Software (BD). Cell proliferation, tracked by dye dilution, was monitored and the percentage of cells in each division was presented.
ELISA cytokine assay
Analysis of lymphocyte proliferation by CFSE enables the functional analysis of lymphocytes because the cells remain alive and the cytokine production profile can be monitored. Culture supernatants were collected at 168h and stored at −80 °C until use. Cytokine levels were titrated using the ELISA method. IL-2, IL-6 and IL-12 concentration were measured by ELISA using monoclonal antibodies according to suppliers’ guidelines (BD Biosciences, PharMingen). IL-22, IL-17A, IL-10 and TGF-β were measured using monoclonal antibodies according to suppliers’ guidelines (e-Bioscences). For the TGF-β measurement the samples were acidified and latent and active cytokine excreted into the culture medium was measured in samples. The plates were read at 450 nm using u-Quant, BD. The mean optical densities (ODs) of triplicate cultures were compared with the standard curves prepared using recombinant cytokines. The detection limit of the assays was 2 pg/ml for IL-6, 8 pg ⁄mL for IL-22, 4 pg ⁄mL for Il-17A, 2 pg/ml for IL-2, 30 pg/ml for IL-10 and 8 pg/ml for TGF-b, 2 pg/ml for IL-12.
MACS sorting of CD4- positive T cells
For negative selection of CD4+ T-cells from MLN ex vivo and after 168h in vitro culture 1 × 107 MLN cells were incubated with a 10 μl mix of antibodies against surface antigens to non-CD4+ T cells for 15 min at 4 °C in PBS/1% BSA and enriched by magnetic field-assisted cell sorting (Dynal Mouse CD4 Negative Isolation Kit, Invitrogen). Purity of the CD4+ cells obtained that way assessed by FACS analysis was better than 96%. Enriched CD4+ cells were stored at −80 °C until further processing.
Analysis of CD4 T cell cycle
After washing the CD4+ T cells in PBS, the resultant pellet was resuspended in 70% ice-cold ethanol with PBS and incubated 20 min on ice. Then the supernatants were aspirated after centrifugation at 500 g for 5 min. The pellet was suspended in 500 μl propidium iodide (PI)-solution in PBS: 50 μg/ml PI 0.1 mg/ml RNase A with 0.05% Triton X-100. The tube was incubated for 40 min at 37 °C. The PI fluorescence of nuclei was measured using a FACSCalibur and analyzed using CELLQUEST PRO software.
Quantitative PCR
Total RNA from CD4 T cells was extracted using the SV Total RNA Isolation System (Promega) according to the manufacturer’s protocol. Thirty ng of total RNA isolated from each samples was then used as a template for cDNA synthesis, prepared with a first-strand cDNA synthesis kit (Thermo Scientific) using TaqMan Gene Expression Assays. Quantitative PCR was performed on the LightCycler Nano Software 1.0 (Roche) specific to mouse survivin (Mm00599749_m1) P27 (Mm00438168_m1) and for the housekeeping gene GAPDH. PCR was performed with TaqMan MGB probes labeled with FAM reporter dye in a final reaction volume of 20 μl. The amplification conditions consisted incubation at 50 °C for 2 min and initial DNA polymerase enzyme activation at 95 °C for 10 min, followed by 50 cycles of denaturation at 95 °C for 15 sec and annealing/extension at 60 °C for 1 min. All PCR reactions were performed in triplicate and validated by the presence of a single peak in the melt curve analysis. The mRNA level for each sample was normalized by dividing the calculated value by the GAPDH value.
Enzyme-linked immunosorbent assay
For the protein detection, CD4 T cells were permabilized with Fixation and Permeabilization Solution (BD, Biosciences). Cyclin D1, P-Protein Pgp, B cell Leukemia/Lymphoma 2 Bcl-2, Bcl-2 Associated X Protein Bax, Survivin levels in CD4 T cells were measured by an Enzyme-linked Immunosorbent Assay Kit according to suppliers’ guidelines (Uscn Life Science Inc.). Because the ratio of Bax to Bcl-2 determines the susceptibility of cells to death signals, the Bax to Bcl-2 ratio was calculated as: Bax/Bcl × 100%.
Caspases activity
Caspase-3 and -8 activity was determined by using a Caspase-3/CPP32 Colorimetric Assay Kit and FLICE/Caspase-8 Colorimetric Assay Kit (Biovision), according to the manufacturer’s instructions. HeLa cells were used as a control for caspase-3 and-8 activity. Relative caspase activity was measured as optical density at 405 nm.
Statistical analyses
The data were collected in three independent experiments. The results and statistical evaluation of a representative experiment were presented. The significance of differences between groups (control and infected) was determined by analysis of variance (ANOVA) using MINITAB Software (Minitab Inc.). All values are expressed as mean ± SE. A P-value < 0.05 was considered to be statistically significant.
Acknowledgments
This research was supported through the National Centre for Research and Development (NN2011/01/M/NZ6/01793). We thank Professor Michael James Stear for advice and help.
Submitted
03/17/2013
Revised
04/30/2013
Accepted
05/10/2013
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/25008
References
- 1.Reddy A, Fried B. The use of Trichuris suis and other helminth therapies to treat Crohn’s disease. Parasitol Res. 2007;100:921–7. doi: 10.1007/s00436-006-0416-4. [DOI] [PubMed] [Google Scholar]
- 2.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med. 2003;197:451–60. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rzepecka J, Lucius R, Doligalska M, Beck S, Rausch S, Hartmann S. Screening for immunomodulatory proteins of the intestinal parasitic nematode Heligmosomoides polygyrus. Parasite Immunol. 2006;28:463–72. doi: 10.1111/j.1365-3024.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 4.Donskow-Łysoniewska K, Majewski P, Brodaczewska K, Jóźwicka K, Doligalska M. Heligmosmoides polygyrus fourth stages induce protection against DSS-induced colitis and change opioid expression in the intestine. Parasite Immunol. 2012;34:536–46. doi: 10.1111/pim.12003. a. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MS, Taylor MD, O’Gorman MT, Balic A, Barr TA, Filbey K, et al. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–96. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donskow-Łysoniewska K, Krawczak K, Doligalska M. Heligmosomoides polygyrus: EAE remission is correlated with different systemic cytokine profiles provoked by L4 and adult nematodes. Exp Parasitol. 2012;132:243–8. doi: 10.1016/j.exppara.2012.07.009. b. [DOI] [PubMed] [Google Scholar]
- 7.Donskow K, Drela N, Doligalska M. Heligmosomoides bakeri antigen rescues CD4-positive T cells from glucocorticoid-induced apoptosis by Bcl-2 protein expression. Parasite Immunol. 2011;33:158–69. doi: 10.1111/j.1365-3024.2010.01262.x. [DOI] [PubMed] [Google Scholar]
- 8.Doligalska M, Donskow-Schmelter K, Rzepecka J, Drela N. Reduced apoptosis in BALB/c mice infected with Heligmosomoides polygyrus. Parasite Immunol. 2007;29:283–91. doi: 10.1111/j.1365-3024.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- 9.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–92. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darzynkiewicz Z, Evenson D, Staiano-Coico L, Sharpless T, Melamed MR. Relationship between RNA content and progression of lymphocytes through S phase of cell cycle. Proc Natl Acad Sci U S A. 1979;76:358–62. doi: 10.1073/pnas.76.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–16. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–9. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 13.Los M, Stroh C, Jänicke RU, Engels IH, Schulze-Osthoff K. Caspases: more than just killers? Trends Immunol. 2001;22:31–4. doi: 10.1016/S1471-4906(00)01814-7. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–4. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 15.Adrain C, Creagh EM, Martin SJ. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001;20:6627–36. doi: 10.1093/emboj/20.23.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton K, Strasser A. Caspases signal not only apoptosis but also antigen-induced activation in cells of the immune system. Genes Dev. 2003;17:819–25. doi: 10.1101/gad.1077403. [DOI] [PubMed] [Google Scholar]
- 17.Tamm I, Wang Y, Sausville ED, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–20. [PubMed] [Google Scholar]
- 18.Kobayashi Y, Yukiue H, Sasaki H, Fukai I, Yokoyama T, Kiriyama M, et al. Developmentally regulated expression of survivin in the human thymus. Hum Immunol. 2002;63:101–7. doi: 10.1016/S0198-8859(01)00369-X. [DOI] [PubMed] [Google Scholar]
- 19.Chen XM, Levine SA, Splinter PL, Tietz PS, Ganong AL, Jobin C, et al. Cryptosporidium parvum activates nuclear factor kappaB in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology. 2001;120:1774–83. doi: 10.1053/gast.2001.24850. [DOI] [PubMed] [Google Scholar]
- 20.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NFkB in development and progression of human cancer. Virchows Arch. 2005;446:475–82. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 21.Jaruga-Killeen E, Rayford W. TNF receptor 1 is involved in the induction of apoptosis by the cyclin dependent kinase inhibitor p27Kip1 in the prostate cancer cell line PC-3. FASEB J. 2005;19:139–41. doi: 10.1096/fj.04-2305fje. [DOI] [PubMed] [Google Scholar]
- 22.Gabet AS, Mortreux F, Talarmin A, Plumelle Y, Leclercq I, Leroy A, et al. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. Oncogene. 2000;19:4954–60. doi: 10.1038/sj.onc.1203870. [DOI] [PubMed] [Google Scholar]
- 23.Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15:491–500. doi: 10.1002/ibd.20787. [DOI] [PubMed] [Google Scholar]
- 24.Baermann G. Eine einfache methods zur anffinolung von Ankylostomum—(Nematoden)— larven in erdproben. Ned Tijdschr Geneeskd. 1917;57:131–7. [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]