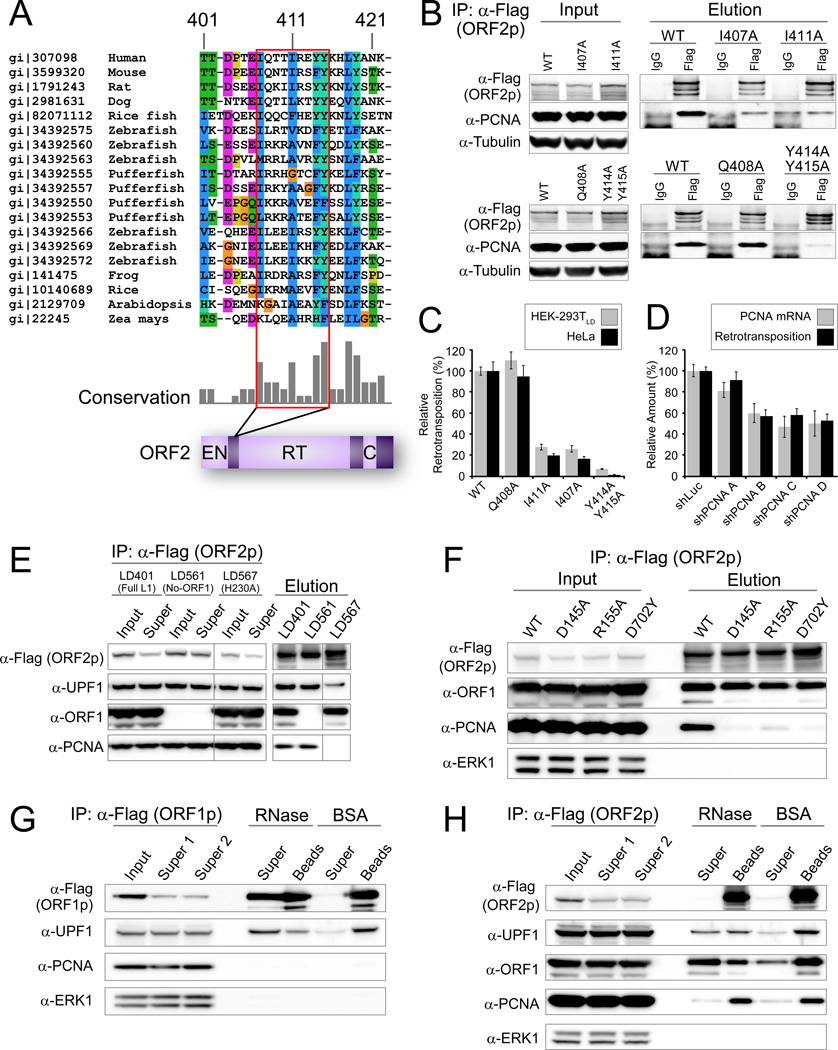
Figure 6. PCNA specifically interacts with conserved region within ORF2p in an EN and RT dependent way.
(A) Partial sequences alignment of LINE ORF2p from various species indicating the PIP box, a known PCNA interaction motif, outlined in red. PIP: QXX(V/L/M/I)XX(F/Y)(F/Y). (B) PCNA specifically co-ips with ORF2p and this interaction is dependent on the PIP box. (C) The PIP box is important for L1 retrotransposition activity in both Hela and HEK293T cells. The retrotransposition efficiency of the wild type L1 was assigned as 1; values are the average of three independent experiments standard error is shown. (D) Knockdown of endogenous PCNA decreased L1 retrotransposition and data are represented as mean +/− SEM. (E) PCNA-ORF2p co-ip is abolished in the ORF2p EN- mutant. UPF1 but not PCNA binding is retained in a mutant ORF2p lacking endonuclease activity (H230A). (F) PCNA-ORF2p co-ip is abolished in an RT- mutant (D702Y) and an additional ORF2p EN- mutant D145A; partially active EN mutant R155A has reduced PCNA recovery. (G) The interaction between UPF1 and ORF1p is RNase sensitive. pLD288 purified with anti-Flag Dynabeads and treated on-bead with RNases A/T1 or mock-treated with BSA. “Super”, supernatants; “Beads” samples retained on beads after washing and eluted with LDS sample buffer. (H) ORF2p–PCNA and ORF2p–ORF2p interactions are RNase resistant, and ORF2p–ORF1p and ORF2p–UPF1 interactions are RNase sensitive. pLD401 material was purified with anti-Flag Dynabeads and treated as in 6G.