Abstract
Monoclonal gammopathy of undetermined significance (MGUS) that presents with no quantifiable M spike on immunofixation electrophoresis (IFE) can be termed IFE MGUS. We retrospectively identified patients with IFE MGUS who were monitored with at least 1 subsequent assessment that included an IFE, and evaluated the persistence of the monoclonal protein and the progression of disease. Although the monoclonal proteins persisted in the majority of patients, 16% did not experience this persistence, and had no documented immunomodulatory therapy. After a median follow-up of 3.9 years, the disease clinically progressed in 14 patients (3.2%). Eight of these 14 patients with clinical progression had an immunoglobulin (Ig) A IFE M protein and 6 had an IgG M protein. This study demonstrates that in some patients with IFE MGUS, the M proteins are transient and that IgA IFE MGUS is more likely to persist and progress to myeloma.
Keywords: MGUS, Progression, Immunofixation electrophoresis
Monoclonal gammopathy of undetermined significance (MGUS) is the most common plasma cell proliferative disorder. MGUS has a prevalence of more than 3% in the population of those aged 50 years or older and the prevalence increases to 5% or higher in those older than 70 years.1 If light-chain MGUS is included, the respective numbers are 4.2% and 6.6%.2 Although asymptomatic, a diagnosis of MGUS has long-term implications for the patient. The overwhelming majority of multiple myeloma cases are preceded by MGUS,3 and the rate of progression of MGUS to multiple myeloma or a related malignant condition is 1% per year.4,5 A serum monoclonal protein concentration of at least 1.5 g/dL (15 g/L) is one of the risk factors for progression.6
To diagnose MGUS, the presence of a monoclonal immunoglobulin must be established by means of immunofixation electrophoresis (IFE). Serum protein electrophoresis (SPEP) is often used as the screening test for detecting monoclonal gammopathies7-9 and will trigger IFE confirmation. SPEP is also a method to quantitate and monitor the M protein; the M protein fraction is described as an M spike. If the concentration of the M protein is small or if the M protein migrates in the β or α fractions, the M protein becomes more challenging to detect.10-13 The limit of detection of M proteins with SPEP is 0.02 to 0.04 g/dL if they migrate in the γ fraction.
The quantitation of M proteins is a different process from the qualitative detection of M proteins. If a small M protein migrates within the β or α fractions or within a large polyclonal background, quantitation of the M protein will include substantial amounts of normal proteins. Quantitating an M spike in which the M protein may be less than 25% of the protein is inaccurate and may be misleading. These small monoclonal proteins are therefore often not quantitated and described only as small abnormalities. Although not quantifiable and more difficult to detect, subtle abnormalities such as fuzzy γ bands can reflect serious diseases. MGUS that presents with no quantifiable M spike but with an IFE abnormality can be termed IFE MGUS.
It is unclear if the outcomes of patients with IFE MGUS are comparable to those with MGUS with small (<1.5 g/dL [15 g/L]) M spikes. The goal of this study was to evaluate our laboratory and clinical experience in terms of persistence and progression of IFE MGUS in patients.
Materials and Methods
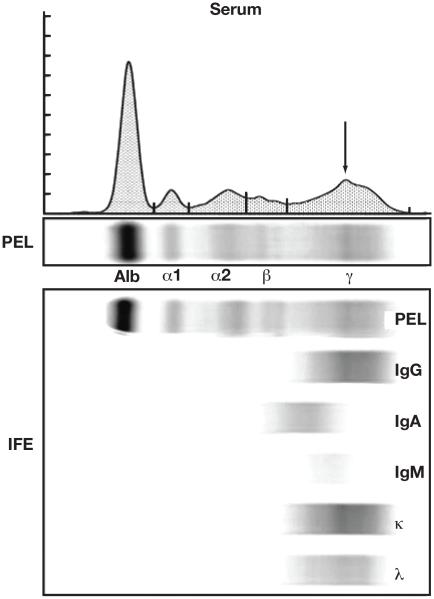
We queried our dysproteinemia database (which contains data on all patients with a plasma cell proliferative disease seen at Mayo Clinic, Rochester, MN) for patients with MGUS whose original entry included a monoclonal intact immunoglobulin (heavy plus light chain) that was detectable on immunofixation but was not quantitated by SPEP and no M-spike value was in the medical record (eg, IFE MGUS). Patients who had biclonal gammopathies, monoclonal free light chain without an intact heavy chain, or indeterminate results were excluded. The IFE MGUS patients had no M-spike quantitation for various reasons: (1) no detectable SPEP abnormality, (2) an elevated β fraction that was symmetric and less than 2 g/dL (20 g/L), and (3) a detectable fuzzy band in the γ fraction that was much smaller than the polyclonal background. Figure 1 illustrates an observable, but nonquantifiable small abnormality in the γ fraction. Although it is possible to quantitate this region of the γ fraction, the majority of the M spike would be the background polyclonal immunoglobulin. Whether to fractionate an M spike in this type of SPEP presentation is a qualitative decision in our laboratory based on the monoclonal immunoglobulin representing at least one third of the M-spike fraction.
Figure 1.
Typical serum protein electrophoresis and immunofixation electrophoresis (IFE) results in a patient with IFE monoclonal gammopathy of undetermined significance arising from a small M protein in the γ fraction. A serum protein electrophoresis gel (PEL) is shown with its electrophoretogram as well as the IFE gel. A small, fuzzy band is visible on the PEL, and is immunotyped as a small monoclonal IgG κ protein. The asymmetry in the γ fraction is indicated on the electrophoretogram with an arrow. If this region of the γ fraction is demarcated as a separate fraction, the monoclonal protein would account for approximately 10% of the area contained in the M spike. Alb, albumin fraction.
We identified 1,820 patients with IFE MGUS. From this population, we excluded any patient who had no further IFE testing at least 1 month after the initial diagnosis. The remaining 437 patients are the basis of this study. The laboratory course for each of the 437 patients was classified as either persistent or nonpersistent. The patients who had invariant positive IFE results were placed into the persistent group, and patients who had at least 1 IFE that did not detect the original clone were classified as nonpersistent. The polyclonal immunoglobulin background in the γ fraction was documented as hypergammaglobulinemic (γ fraction >1.6 g/dL [16 g/L]), normal (γ fraction 0.6-1.6 g/dL [6-16 g/L]), and hypogammaglobulinemic (γ fraction <0.6 g/dL [6 g/L]). The clinical records of the patients were examined for adverse outcomes, and the time to progression was documented. For the nonpersistent cohort, the electronic medical records were reviewed to see if an immunosuppressant therapy or immunologic disease was present during the time of the study. All queries of patient medical records followed a protocol approved by the Mayo Clinic institutional review board.
All assays were performed according to protocols in the clinical immunology laboratory: SPEP was performed on the SPIFE SPE system (Helena Laboratories, Beaumont, TX) and IFE on Sebia 9IF gels (Sebia, Norcross, GA). The total protein concentration was determined with a colorimetric assay using biuret reagents from Roche and a Roche Hitachi 912 chemistry analyzer system (Roche Diagnostics, Indianapolis, IN).
A χ2 statistic was calculated to assess whether distributions of persistence and progression were dependent on immunoglobulin class or nature of the polyclonal immunoglobulin at the time of presentation.
Results
The database query yielded a total of 1,820 patients whose first diagnosis was an IFE MGUS. Of these 1,820 patients with IFE MGUS, 437 (24%) had follow-up studies that included another IFE at least 1 month after the original IFE. Table 1 summarizes the characteristics of all 1,820 patients with IFE MGUS by comparing the subset of 437 patients with at least 1 subsequent IFE to the 1,383 patients with no IFE follow-up. The sex, age, and heavy-chain isotype distribution were similar in the 2 groups. The γ fraction classifications at presentation were different in the patients with IFE MGUS who had IFE monitoring from those who had no follow-up IFE (P < .001). Among the patients with follow-up, 91% presented with normal γ globulin concentrations, 8% with a hypergammaglobulinemic background, and 1% with a hypogammaglobulinemic background. In patients with no IFE follow-up, the γ fraction distribution was as follows: normal, 83%; hypergammaglobulinemic, 16%; and hypogammaglobulinemic, 1%.
Table 1.
Characteristics of the Populations With IFE MGUS*
| Patients With No Follow-up IFE |
Patients With at Least 1 Follow-up IFE |
P
Value |
|
|---|---|---|---|
| No. of patients | 1,383 | 437 | |
| Sex | |||
| M | 812 (59) | 243 (56) | .40 |
| F | 571 | 194 | |
| Median (range) age, y | 69 (21-99) | 67 (22-96) | .40 |
| Class | .44 | ||
| IgG | 865 (63) | 256 (59) | |
| IgA | 238 (17) | 93 (21) | |
| IgM | 279 (20) | 87 (20) | |
| IgD | 1 (<1) | 1 (<1) | |
| γ fraction | .006 | ||
| Hypogammaglobulinemic | 9 (<1) | 4 (1) | |
| Normal γ fraction | 1,152 (83) | 396 (91) | |
| Hypergammaglobulinemic | 222 (16) | 37 (8) | |
| Clinical follow-up, y | |||
| Mean | 4.8 | ||
| Median | 3.9 | ||
| Range | 0.2-13 | ||
| Patients with clinical progression |
14 |
IFE immunofixation electrophoresis; Ig, immunoglobulin; MGUS, monoclonal gammopathy of undetermined significance.
Data are given as number (percentage) unless otherwise indicated.
Follow-up testing in which IFE was ordered indicated that 70% of patients had a result consistent with the original finding Table 2. Thirty percent of the IFE MGUS proteins either disappeared or had inconsistent results at some time during follow-up. Persistence was found to be dependent on the immunoglobulin class (P < .001) but independent of the original γ fraction classification (P = .40). The immunoglobulin (Ig) A IFE M proteins persisted in 87% of the cases whereas only 64% of IgG and 73% of IgM IFE M proteins persisted. There was no difference in persistence between presentation in a normal γ fraction and a hypergammaglobulinemic background.
Table 2.
Persistence and Progression in Patients With IFE MGUS
| No. of Patients |
Persistence, % |
Progression, No. (%) |
|
|---|---|---|---|
| All patients | 437 | 70 | 14 (3.2) |
| IgG | 256 | 64 | 6 (2.3) |
| IgA | 93 | 87 | 8 (8.6) |
| IgM | 87 | 73 | 0 |
| IgD | 1 | 100 | 0 |
| Normal γ fraction | 396 | 70 | 12 (3.0) |
| Hypergammaglobulinemic | 37 | 72 | 2 (5.4) |
| Hypogammaglobulinemic | 4 | 100 | 0 |
IFE immunofixation electrophoresis; Ig, immunoglobulin; MGUS, monoclonal gammopathy of undetermined significance.
The median laboratory follow-up period from first IFE to last IFE was 2.6 years and the median clinical follow-up from first IFE to last day seen in our clinic was 3.9 years (range, 0.2-13 y) (Table 1). A total of 14 patients (3.2%) had clinical progression to a hematologic disease (Table 2). Progression was found to be dependent on the immunoglobulin class (P = .002). Eight of the 14 (57%) patients in whom the disease clinically progressed had an IgA IFE M protein while none had an IgM IFE M protein. The patients with clinical progression are listed in Table 3.
Table 3.
Type of Clinical Progression in Patients With IFE MGUS
| Disease | Sex | Ig Class | Time to Progression, y |
|---|---|---|---|
| Multiple myeloma | M | IgA | 1.0 |
| F | IgG | 2.8 | |
| M | IgA | 9.9 | |
| M | IgA | 2.1 | |
| M | IgG | 3.5 | |
| F | IgG | 1.7 | |
| F | IgA | 1.3 | |
| F | IgA | 2.5 | |
| Smoldering myeloma | M | IgA | 5.1 |
| M | IgA | 4.5 | |
| Primary amyloidosis | M | IgG | 4.5 |
| Light chain deposition disease | F | IgG | 8.9 |
| Extramedullary myeloma | F | IgG | 0.4 |
| Lymphoplasmacytic lymphoma | F | IgA | 5.6 |
IFE immunofixation electrophoresis; Ig, immunoglobulin; MGUS, monoclonal gammopathy of undetermined significance.
The medical records of the 129 nonpersistent patients with IFE MGUS were reviewed for possible causes of the nonpersistence Table 4. The review indicated that 71 (55%) of the nonpersistent patients with IFE MGUS had documented therapy or an immunologic disorder that could account for the inconsistency in the detection of the monoclonal immunoglobulin. Excluding these 71 patients resulted in 366 IFE MGUS patients with follow-up and 58 nonpersistent monoclonal gammopathies (16%). The immunoglobulin class distribution of the 58 nonpersistent patients was as follows: IgA, 5% (3/58); IgM, 26% (15/58); and IgG, 71% (41/58). This nonpersistence cohort was found to be significantly different in immunoglobulin class distribution from the study population (P < .001).
Table 4.
Documented Immunomodulating Treatments in the Nonpersistent Group (n = 129)
| Condition | No. |
|---|---|
| Hematologic malignancy | |
| MDS transformation | 4 |
| Chronic lymphocytic leukemia | 1 |
| Chronic myelomonocytic leukemia | 1 |
| Hairy cell leukemia | 1 |
| Solid organ tumor treated with chemotherapy | 2 |
| Plasmacytoma irradiated | 2 |
| Documented plasma cell disorder outside study time frame | 2 |
| After solid organ transplantation | 38 |
| Autoimmune disease with DMARD treatment | 17 |
| Lymphocytic thymoma with prednisone treatment | 1 |
| HIV infection | 2 |
| Total | 71 |
DMARD, disease-modifying antirheumatic drug; MDS, myelodysplastic.
Discussion
We retrospectively studied 437 patients with IFE MGUS having clinical follow-up and laboratory tests that included IFE. Although 1,820 patients with IFE MGUS were seen, most physicians requested only SPEP to monitor patients. In the absence of repeat IFE tests, these patients were excluded from the study cohort because persistence of the M protein could not be definitively documented for this analysis. As illustrated in Table 1, the characteristics of the 1,383 patients who were not monitored by IFE do not appear different by sex, age, or isotype distribution. This group was enriched, however, for patients with hypergammaglobulinemia, and this may reflect the view that small monoclonal proteins in hypergammaglobulinemic backgrounds are not as clinically significant as small monoclonal proteins in normal or hypogammaglobulinemic backgrounds.
In a previous study of the risk of MGUS progression to myeloma and related diseases,3 the median size of the M spike was 1.3 g/dL (13 g/L). That study concluded that the risk of progression was 1% per year, and in addition, larger M spikes predicted higher progression rates. As SPEP methods have improved from cellulose acetate to agarose and high resolution capillary electrophoresis, and as screening panels have added IFE, the sensitivity for detecting monoclonal gammopathies has also improved. In the current study, we examined patients with MGUS whose M proteins are deemed too small to quantitate and thus whose median M spike is not measurable. The distribution of immunoglobulin heavy chains in the patients with IFE MGUS was as follows: IgG, 59%; IgA, 21%; and IgM, 19%. This differs from a prevalence study that used SPEP to screen residents in Olmsted County, MN, and found a distribution of 69% IgG, 11% IgA, and 17% IgM.1 In that study, 13% of the participants had IFE MGUS and 87% had a measurable M spike. The increase in IgA MGUS compared with the original prevalence study is partially because of the higher portion of IgA monoclonal proteins that migrate in the β region of the electrogram and which were not visible in the SPEP screen.
In the absence of treatment, monoclonal gammopathies typically persist or progress. In a study looking at long-term prognosis for MGUS the protein disappeared without an apparent cause or intervention in only 0.4% of patients with a quantifiable monoclonal protein.4 In the current study, all of the patients had monoclonal protein concentrations that were too small to quantitate, and 30% became undetectable on follow-up. Table 3 lists the type of immune modifying treatments in the nonpersistent group that might explain the disappearance of the monoclonal proteins. Among the nonpersistent monoclonal protein samples, 30% were from patients with solid organ transplants. This is a common experience for such patients. Previously published data showed that transient monoclonal or oligoclonal gammopathies occur after bone marrow or solid organ transplantation.14,15 Excluding the patients with documented immune-modifying treatments resulted in 16% of patients with IFE MGUS having M proteins that were not persistent. It is likely that some low-concentration M proteins that are detected on IFE are the result of a transient clonal proliferation caused by an infection or autoimmune disease and are therefore qualitatively different from monoclonal gammopathies. If these transient clones represent the most abundant clone in a polyclonal proliferation, we would expect small monoclonal proteins found in hypergammaglobulinemic patients to be less persistent than those within a normal γ fraction. This was not the case; small monoclonal proteins were just as likely to persist in normal as in hypergammaglobulinemic backgrounds. The small number of M proteins presenting in the hypogammaglobulinemic backgrounds, however, all persisted.
In spite of the disappearance of the monoclonal protein in 16% of the patients with IFE MGUS, in some patients with persistent IFE MGUS the disease progressed to plasma cell malignancy. During a median of 3.9 years, the disease progressed in 14 patients with IFE MGUS. Progression rates were found to correlate with the immunoglobulin class. The odds ratio of progression for IgA compared with IgG was 3.7. This suggests that IgG and IgA IFE MGUS patient cohorts are not qualitatively different from the MGUS patient cohorts previously described, in which patients with IgA MGUS were at higher risk for progression than those with IgG MGUS.4 It is interesting that we did not document progression in any patient with IgM IFE MGUS, despite the fact that 20% of the patients with IFE MGUS had IgM gammopathy.
We caution against overinterpretation of the progression data because ours is a small retrospective study that was not designed for diligent, long-term follow-up. Three fourths of the 1,820 patients with IFE MGUS did not receive a subsequent follow-up IFE test. In Table 1 we indicate that sex, age, and heavy-chain isotype were not different from the 437 patients who were monitored with IFE. We do not know, however, if these patients had no follow-up, had clinical follow-up with no laboratory evaluation, or were monitored with SPEP but not IFE. If any patient had been monitored and experienced progression to multiple myeloma or a related disease, we assume SPEP and IFE would then have been requested. In spite of this shortcoming, the results of this study demonstrate that (1) approximately 16% of IFE MGUS cases are transient; (2) IgA IFE MGUS is more likely to be persistent; and (3) IgA IFE MGUS is associated with a greater rate of progression than IgG and IgM.
Acknowledgments
This study was supported in part by the National Cancer Institute (CA107476, CA100707, CA 83724), National Institutes of Health, US Public Health Service, Bethesda, MD, and in part by the Jabbs Foundation, Birmingham, England, and the Henry J. Predolin Foundation, Madison, WI.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–1728. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA. Monoclonal gammopathy of undetermined significance: natural history in 241 cases. Am J Med. 1978;64:814–826. doi: 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–817. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keren DF, Alexanian R, Goeken JA, et al. Guidelines for clinical and laboratory evaluation patients with monoclonal gammopathies. Arch Pathol Lab Med. 1999;123:106–107. doi: 10.5858/1999-123-0106-GFCALE. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 9.Katzmann JA. Screening panels for monoclonal gammopathies: time to change. Clin Biochem Rev. 2009;30:105–111. [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshminarayanan R, Li Y, Janatpour K, et al. Detection by immunofixation of M proteins in hypogammaglobulinemic patients with normal serum protein electrophoresis results. Am J Clin Pathol. 2007;127:746–751. doi: 10.1309/QJ3PY18PMMJ8AYEH. [DOI] [PubMed] [Google Scholar]
- 11.Katzmann JA, Stankowski-Drengler TJ, Kyle RA, et al. Specificity of serum and urine protein electrophoresis for the diagnosis of monoclonal gammopathies. Clin Chem. 2010;56:1899–1900. doi: 10.1373/clinchem.2010.152280. [DOI] [PubMed] [Google Scholar]
- 12.Narayan S, Lujan MG, Baskin LB, et al. Measurement of beta1- and beta2-globulins improves detection of M-spikes on high-resolution electrophoresis. Clin Chem. 2003;49:676–678. doi: 10.1373/49.4.676. [DOI] [PubMed] [Google Scholar]
- 13.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 14.Mitus AJ, Stein R, Rappeport JM, et al. Monoclonal and oligoclonal gammopathy after bone marrow transplantation. Blood. 1989;74:2764–2468. [PubMed] [Google Scholar]
- 15.Radl J, Valentin RM, Haaijman JJ, et al. Monoclonal gammopathies in patients undergoing immunosuppressive treatment after renal transplantation. Clin Immunol Immunopathol. 1985;37:98–102. doi: 10.1016/0090-1229(85)90140-0. [DOI] [PubMed] [Google Scholar]