Figure 1. Schematic diagram of the roles of DA-AC-cAMP-PKA and NO-sGC-cGMP-PKG signaling and PDE function in the regulation of MSN membrane excitability.
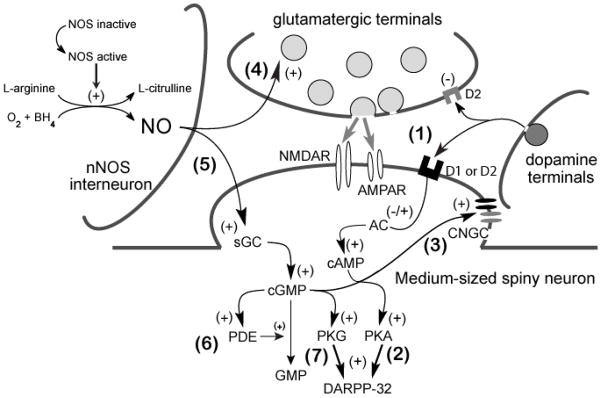
(1) DA released from DA terminals binds to D1-like or D2-like DA receptors on the postsynaptic MSN and leads to either stimulation or inhibition of AC, respectively. (2) Increases in cAMP tone will activate PKA which can phosphorylate DARPP-32 to have complex effects on downstream signaling pathways, neurotransmitter receptors, and voltage-gated ion channels (see [1] for review). (3) cAMP (along with cGMP) can also stimulate CNGCs in the plasma membrane which can lead to cation influx and membrane depolarization. (4) Tonic NO signaling increases glutamatergic transmission across corticostriatal synapses potentially via nitrosylation of presynaptic release proteins or a sGC-cGMP-dependent mechanism. (5) NO transmission can also activate sGC in the postsynaptic MSN and stimulate cGMP production. cGMP can activate CNGC (see step 3), stimulate PDEs (6), or activate PKG (7). Transient activation of NO-sGC-cGMP signaling in the intact striatum increases the responsiveness of MSNs to excitatory glutamatergic drive and facilitates short-term potentiation of corticostriatal synaptic transmission (see above and [57] for review). More robust stimulation of NO-sGC-cGMP-PKG signaling performed in striatal slice preparations can induce LTD of corticostriatal synaptic transmission (see [22] for review). Together these studies suggest that like DA D1/5 receptor modulation, the net impact of NO-sGC-cGMP-PKG signaling on membrane excitability may depend on the steady-state membrane potential of MSNs and interactions with glutamateric drive and NMDA receptor activation (Modified with permission from [57]).
