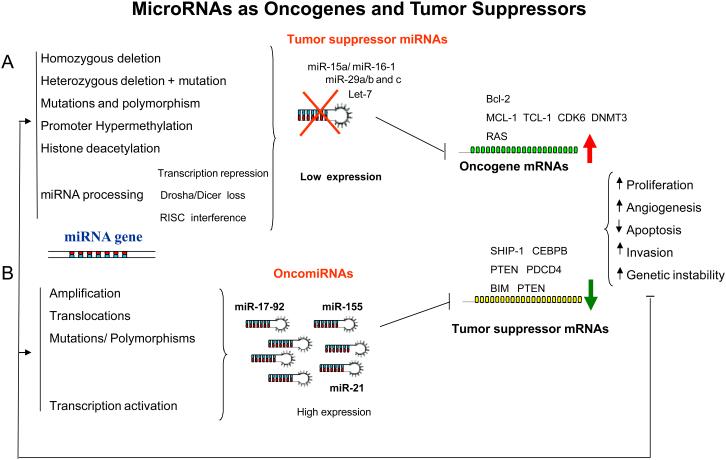
Figure 2. MicroRNAs as oncogenes and tumor suppressors.
(A) In this model, we propose that a miRNA that normally downregulates an oncogene can be defined as a tumor suppressor gene, and is often lost in tumor cells. The loss of function of this miRNA by mutation, deletion, promoter methylation or any abnormalities in the miRNA biogenesis might result in an abnormal expression of the target oncogene, which subsequently contributes to tumor formation by inducing cell proliferation, invasion, angiogenesis and decreased cell death. Some of the proposed mechanisms for inactivation of miRNAs in cancer are experimentally proven, such as the down-regulation of miR-15a/miR-16-1 expression in CLL patients that harbor homozygous and heterozygous deletions at 13q14.3, where the miR-15a/miR-16–1 cluster is located23 and the loss of miR-29b-1/miR-29a cluster in AML patients with 7q- (This cluster is located in 7q32)63. In addition, germ-line mutations were found in the miR-15a/miR-16–1 precursor that resulted in lower miR-15a and miR-16-1 expression levels. Overall, the loss of both miR-15a/miR-16-1 and miR-29b-1/miR-29a cluster results in up-regulation of target oncogenes like BCL-2, MCL-1, TCL-1, CDK6 and DNMT3a61,41,63,119,125. (B) The amplification or overexpression of a miRNA that downregulates a tumor suppressor or other important genes involved in differentiation might contribute to tumor formation by stimulating proliferation, angiogenesis and invasion and preventing apoptosis and increasing genetic instability. For example, amplifications of the oncogenic miRNAs, miR-17–92 cluster, miR-21 and miR-155 have been clearly associated with tumor initiation and progression by repressing the expression of tumor suppressor genes like PTEN, BIM and PDCD427,67-68,70. The impact of the aberrant miRNA expression on the transcriptome and proteome will result in increased cell proliferation, angiogenesis, invasion, anti-apoptosis and genomic instability, which in turn will damage further the genome, perpetuating a dangerous cycle. For example, increased genomic instability may predispose for more mutations that may induce cancer progression or refractoriness to treatment.