Fig. 4. LPA promotes co-localization of TRIP6 with the LPA2 receptor and TRIP6 targeting to focal adhesions.
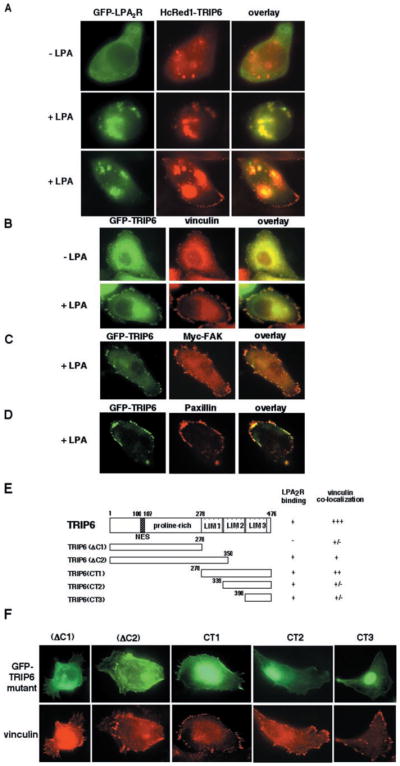
A, SKOV3 cells were co-transfected with the expression plasmids of GFP-LPA2R and HcRed1-TRIP6. After serum starvation overnight, cells were treated without or with 2 μM LPA for 10 min. The GFP fluorescence of LPA2R and the far red fluorescence of TRIP6 were visualized directly by fluorescence microscopy. B–D, SKOV3 cells transiently expressing GFP-TRIP6 alone or with Myc-FAK were treated without or with 2 μM LPA for 15 min. Cells were fixed, and GFP-TRIP6 was visualized by fluorescence microscopy. The immunostaining of endogenous vinculin (B), Myc-FAK (C), and endogenous paxillin (D) was performed as described under “Experimental Procedures.” E, the molecular structures of different TRIP6 truncation mutants and a summary of their capacity to bind to the LPA2 receptor and co-localize with vinculin. F, SKOV3 cells expressing GFP-TRIP6 mutants were starved overnight and treated with 2 μM LPA for 15 min. The endogenous vinculin was detected with an anti-vinculin antibody, and GFP-TRIP6 mutants were visualized by fluorescence microscopy.
