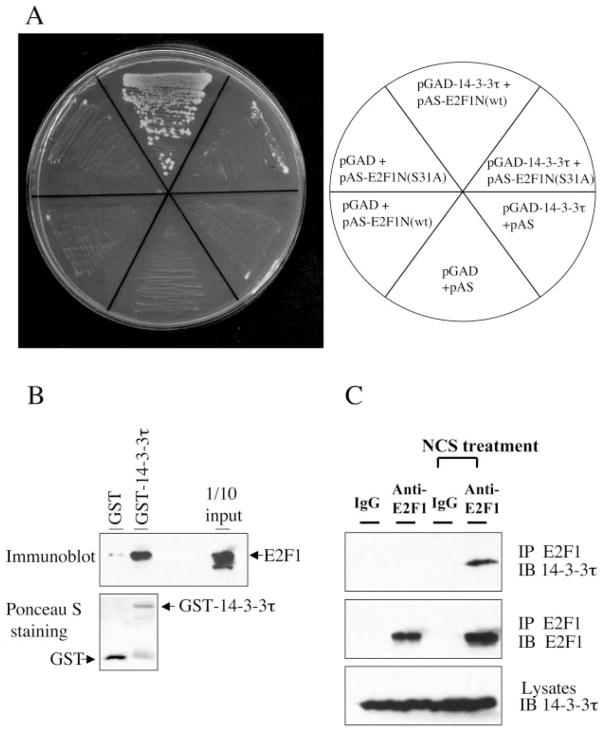
Fig. 1. Interaction of E2F1 and 14-3-3τ in vitro and in vivo.
A, E2F1 interacts with 14-3-3τ in yeast. The interaction between the E2F1 N terminus (amino acids 1–109; named E2F1N) and 14-3-3τ in Saccharomyces cerevisiae Y190 cells was verified by selective growth of transformants on a plate lacking tryptophan, leucine, and histidine and supplemented with 3-amino-1,2,4-triazole. Mutation at the Ser31 to alanine (S31A) inhibits the interaction. B, interaction of E2F1 and 14-3-3τ in vitro. Purified GST or GST-14-3-3τ was incubated with E2F1 protein, and a GST pull-down assay was performed. E2F1 protein was detected by immunoblotting. GST-14-3-3τ and GST were visualized by Ponceau S staining (bottom). C, interaction of endogenous E2F1 and 14-3-3τ during DNA damage. 293T cells were left untreated or treated with the radiomimetic agent NCS at 300 ng/ml for 3 h. Lysates were immunoprecipitated (IP) with normal rabbit IgG or rabbit anti-E2F1 antibody and immunoblotted (IB) as indicated. An aliquot of lysates was probed with anti-14-3-3τ antibody (bottom).