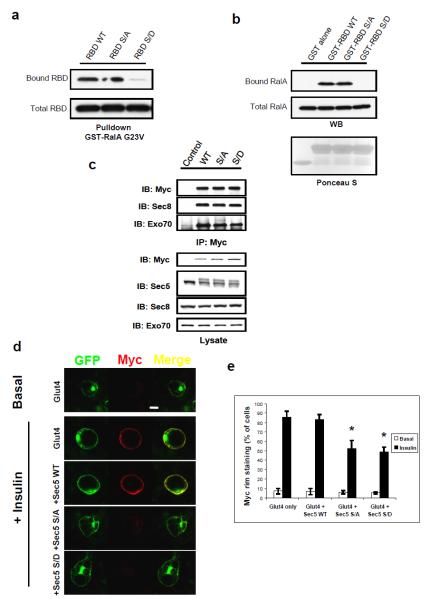
Figure 3. Sec5 effector domain phosphorylation disengages the G protein RalA during vesicle targeting.
(a) The phosphorylation mimetic substitution S89D inhibits Sec5 RBD binding to active RalA. Cos-1 cells expressing the indicated RBD constructs were lysed and subjected to pull down with immobilized GST-RalA G23V. RBD bound to RalA or present in total cell lysates were determined by WB. (b) Direct inhibition of RalA interaction by phospho-mimetic S89D RBD. Recombinant RBD of the indicated forms were purified from E.coli and immobilized on GSH beads before being used to pull down endogenous RalA from cell lysates. RalA bound to immobilized RBD or present in total cell lysates was determined by WB. (c) Replacement of endogenous Sec5 by exogenous copies of the protein. Adipocytes were infected with lentivirus expressing the indicated Myc-tagged Sec5 for 3 weeks before being subjected to cell lysis and IP with Myc antibody. IPs and total cell lysates were fractioned by SDS-PAGE and were subjected to WB analysis with indicated antibodies. Arrows indicated exogenous Sec5 (upper band) and endogenous Sec5 (lower band). (d) Perturbation of Sec5 Ser89 phosphorylation disrupts plasma membrane fusion of Glut4. Adipocytes were co-infected with lentivirus expressing the indicated Sec5 constructs and the Myc-Glut4-eGFP reporter at a 6:1 ratio for 3 weeks. Cells were starved for 4 hours before stimulation with vehicle or 100ng/μl insulin for 20 minutes, fixed with 10% formalin for 10 minutes without permeablization before being subjected to immunostaining with Myc (red) antibody and confocal microscopy. Images were at the same magnification; bar = 10μm. (e) Quantification of five different experiments (n=5) performed as in (d). A minimum of 400 cells in total were counted for each situation. The error bars represent SD. Asterisk: p<0.01.