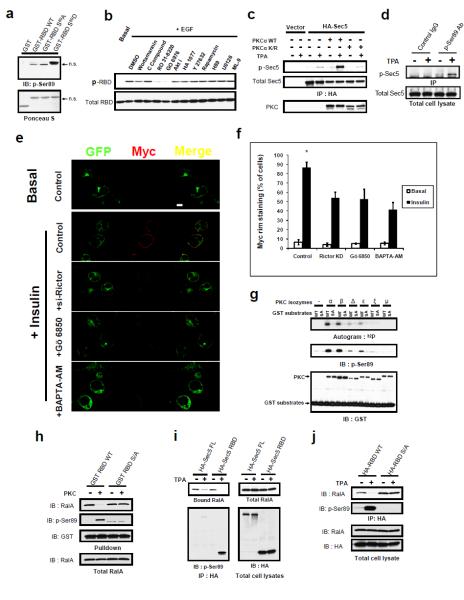
Figure 4. Protein Kinase C (PKC) catalyzes Sec5 effector domain phosphorylation that negatively regulates RalA interaction.
(a) Recognition of the phospho-mimetic S89D substitution by p-Ser89 antibody. Indicated GST fusion proteins purified from E.coli were blotted with p-Ser89 antibody after SDS-PAGE. (b) PKC inhibitors abolish Sec5 RBD Ser89 phosphorylation. Cos-1 cells expressing HA-tagged Sec5 RBD were treated with the indicated inhibitors. Phosphorylation was detected with the p-Ser89 antibody after anti-HA IP. (c) PKCα stimulates Sec5 Ser89 phosphorylation in vivo. Cos-1 cells expressing the indicated constructs were stimulated as described. Phosphorylation was detected with p-Ser89 antibody after anti-HA IP. (d) TPA induces phosphorylation of endogenous Sec5. Cos-1 cells were stimulated as in (b) before subjected IP and WB with the indiated antibody. (e) Loss of PKC activity inhibits Glut4 exocytosis. 3T3-L1 adipocytes expressing Myc-Glut4-eGFP reporter were transfected with control siRNA or Rictor siRNA, or treated with Gö6850 or BAPTA-AM to inhibit PKC activity. Cells were processed as in (2j) for confocal microscopy. Images were of the same magnification; bar = 10μm. (f) Quantification of four independent experiments (n=4) performed as in (e). A total of 400 cells were counted for each situation. Errors represent SD. Asterisk: p< 0.001. (g) Direct phosphorylation of Sec5 Ser89 by PKC isozymes. GST fragments containing Sec5 Ser89 (or S89A) were incubated with the indicated PKC isozymes. Phosphorylation was detected by 32P incorporation and p-Ser89 WB. (h) PKC phosphorylation directly inhibits RalA interaction with Sec5 RBD. Wild type or S89A GST-Sec5 RBD was incubated without or with recombinant PKCα before immobilized on GSH beads, then used to pull down endogenous RalA from cell lysates. (i) PKC phosphorylates Sec5 to inhibit RalA interaction in vivo. Cos-1 cells were transfected with HA-tagged Sec5 full length (FL) or RBD, FLAG-RalA G23VK47E, and wild type GST-PKCα. were subjected to anti-HA IP antibody after the indicated stimulation. (j) Ser89 is required for phosphorylation dependent disengagement of RalA-Sec5 interaction in vivo. Cos-1 cells were transfected with HA-tagged wild type Sec5 or S89A RBD, FLAG-RalA G23V, and GST-PKCα were stimulated as in (c), and subjected to IP and WB with the indicated antibodies.