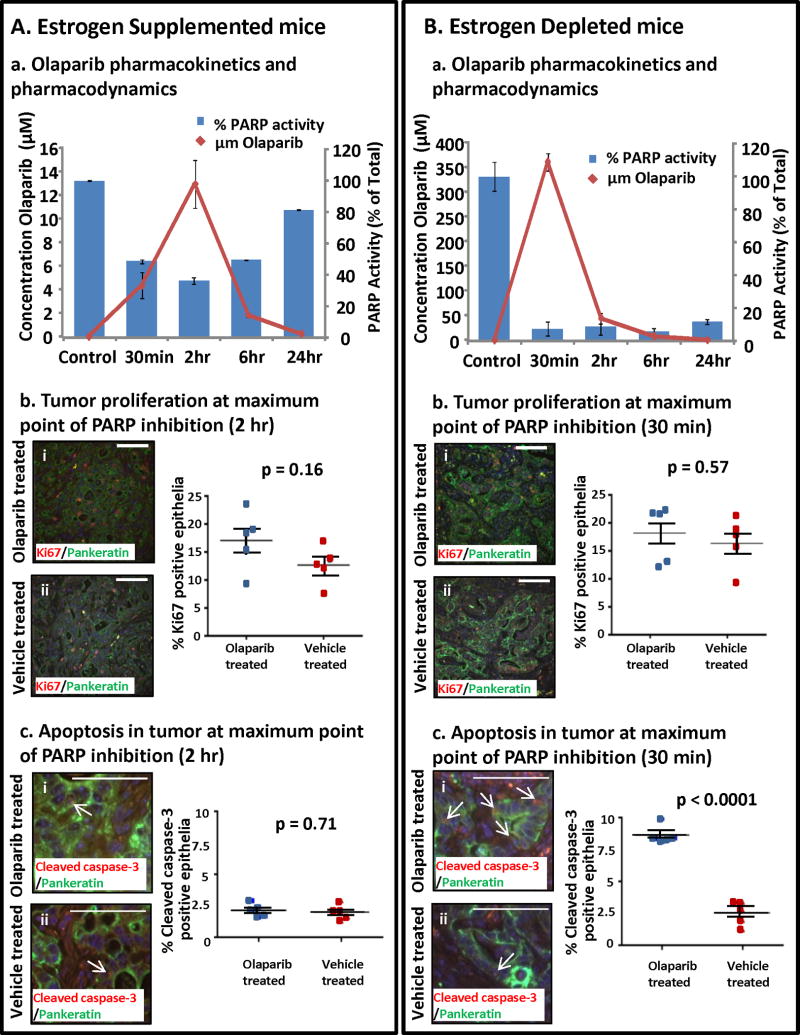
Figure 4. Pharmacokinetics and pharmacodynamics of Olaparib varied with circulating estrogen levels.
(A) Olaparib was detected in the serum of estrogen supplemented mice with measurable inhibition of PARP enzyme activity but no apparent effects on tumor proliferation or apoptosis. (a) Olaparib serum concentrations were undetectable in vehicle treated mice, peaked at 2 h after drug ingestion and then slowly returned to baseline levels. Inhibition of PARP enzyme activity at the tumor bed correlated with the Olaparib serum concentration. (b) Epithelial proliferation (pankeratin & Ki67 dual positive cells) in Olaparib and vehicle treated tumors was similar (i vs. ii, p = 0.16). (c) PARP inhibition in a high estrogenic milieu did not increase apoptotic cell death in tumor PTEN-null epithelia (pankeratin & cleaved caspase 3 dual positive cells) (i vs ii, p=0.71). (B) Higher serum Olaparib levels concomitant with sustained PARP inhibition was detected in estrogen deprived tumor bearing mice. (a) In estrogen depleted mice peak concentrations of serum Olaparib were detected 30 min after dosing then dropped to baseline within 24 h. PARP activity at the tumor bed was sharply decreased compared to vehicle treated mice, and PARP inhibition was sustained throughout a 24h period. (b) PARP inhibition did not diminish epithelial proliferation in estrogen depleted mice (i vs. ii, p = 0.57). (c) Increased apoptosis was observed in tumors subjected to PARP inhibition with estrogen deprivation (I vs. ii, p<0.0001). Scale bars equal 100 μm.