Abstract
An elevated level of fibroblast growth factor-23 (FGF-23) is the earliest abnormality of mineral metabolism in CKD. High FGF-23 levels promote left ventricular hypertrophy but not coronary artery calcification. We used survival analysis to determine whether elevated FGF-23 is associated with greater risk of adjudicated congestive heart failure (CHF) and atherosclerotic events (myocardial infarction, stroke, and peripheral vascular disease) in a prospective cohort of 3860 participants with CKD stages 2–4 (baseline estimated GFR [eGFR], 44±15 ml/min per 1.73 m2). During a median follow-up of 3.7 years, 360 participants were hospitalized for CHF (27 events/1000 person-years) and 287 had an atherosclerotic event (22 events/1000 person-years). After adjustment for demographic characteristics, kidney function, traditional cardiovascular risk factors, and medications, higher FGF-23 was independently associated with graded risk of CHF (hazard ratio [HR], 1.45 per doubling [95% confidence interval (CI), 1.28 to 1.65]; HR for highest versus lowest quartile, 2.98 [95% CI, 1.97 to 4.52]) and atherosclerotic events (HR per doubling, 1.24 [95% CI, 1.09 to 1.40]; HR for highest versus lowest quartile, 1.76 [95% CI, 1.20 to 2.59]). Elevated FGF-23 was associated more strongly with CHF than with atherosclerotic events (P=0.02), and uniformly was associated with greater risk of CHF events across subgroups stratified by eGFR, proteinuria, prior heart disease, diabetes, BP control, anemia, sodium intake, income, fat-free mass, left ventricular mass index, and ejection fraction. Thus, higher FGF-23 is independently associated with greater risk of cardiovascular events, particularly CHF, in patients with CKD stages 2–4.
CKD is an international public health epidemic that increases risk of premature death due to cardiovascular disease.1–4 Rates of atherosclerotic disease are high in CKD, but risk of congestive heart failure is even more striking, with hazards approximately 3-fold higher than in non-CKD populations.5,6 Excess risk of congestive heart failure in CKD is often attributed to hypertension and anemia.6–9 However, aggressive control of these risk factors has not significantly improved heart failure outcomes in patients with CKD, suggesting additional mechanisms of disease.10–12
Fibroblast growth factor-23 (FGF-23) is secreted by osteocytes and regulates phosphate and vitamin D homeostasis by stimulating phosphaturia and inhibiting activation of vitamin D in the kidney.13 FGF-23 levels rise as kidney function declines, and higher levels are strongly associated with greater risk of death.14–19 As a potential explanatory mechanism of FGF-23–associated mortality, multiple studies consistently demonstrated that higher FGF-23 levels are independently associated with greater risk of prevalent and incident left ventricular hypertrophy,20–22 which is an important mechanism of cardiovascular disease in patients with CKD.8 In support of a causal role for elevated FGF-23 in the pathogenesis of left ventricular hypertrophy, FGF-23 stimulated pathologic hypertrophy of isolated cardiac myocytes and induced left ventricular hypertrophy in animals, independent of BP.20 In contrast, observational studies reported conflicting results on the association of FGF-23 with arterial calcification, which is another prominent pattern of cardiovascular injury in CKD.23 In the largest study to date, FGF-23 was not independently associated with coronary artery calcification in patients with CKD stages 2–4,24 and laboratory studies failed to demonstrate a procalcification effect of FGF-23 on vascular smooth muscle cells.24,25 These data suggest that direct effects of FGF-23 on cardiac remodeling, rather than the arterial vasculature, may underlie its association with mortality. We tested the hypotheses that elevated FGF-23 is a risk factor for cardiovascular disease events in patients with CKD stages 2–4 enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study, and that FGF-23 is more strongly associated with risk of congestive heart failure compared with atherosclerotic events.
Results
The study included 3860 participants with CKD from the CRIC study. Mean age of study participants (±SD) was 58±11 years, the mean estimated GFR (eGFR) was 44±15 ml/min per 1.73 m2, and median FGF-23 was 145.4 RU/ml (interquartile range, 96.0–238.8 RU/ml). Traditional cardiovascular risk factors were more prevalent in those with higher FGF-23 levels (Table 1).
Table 1.
Baseline characteristics of the study population by quartiles of FGF-23
| Characteristic | FGF-23 Quartile | P Value | |||
|---|---|---|---|---|---|
| <96.0 RU/ml (n=964) | 96.0–145.4 RU/ml (n=966) | 145.5–238.9 RU/ml (n=965) | ≥239.0 RU/ml (n=965) | ||
| Demographics | |||||
| Age (yr) | 56.1±11.2 | 58.2±10.9 | 58.5±10.8 | 57.8±10.9 | <0.001 |
| Women | 347 (36.0) | 383 (39.7) | 453 (46.9) | 544 (56.4) | <0.001 |
| Race/ethnicity | <0.001 | ||||
| Non-Hispanic white | 450 (46.7) | 419 (43.4) | 402 (41.7) | 344 (35.7) | |
| Non-Hispanic black | 399 (41.4) | 369 (38.2) | 382 (39.6) | 450 (46.6) | |
| Hispanic | 71 (7.4) | 136 (14.1) | 136 (14.1) | 149 (15.4) | |
| Other | 44 (4.6) | 42 (4.4) | 45 (4.7) | 22 (2.3) | |
| Annual income <$20,000a | 184 (19.1) | 268 (27.7) | 335 (34.7) | 410 (42.5) | <0.001 |
| Medical history | |||||
| Hypertension | 727 (75.4) | 839 (86.9) | 867 (89.8) | 889 (92.1) | <0.001 |
| Diabetes | 290 (30.1) | 440 (45.6) | 549 (56.9) | 592 (61.4) | <0.001 |
| Prior atherosclerotic CVD | 198 (20.5) | 275 (28.5) | 329 (34.1) | 377 (39.1) | <0.001 |
| Myocardial infarction or revascularization | 144 (14.9) | 209 (21.6) | 233 (24.2) | 262 (27.2) | <0.001 |
| Stroke | 76 (7.9) | 79 (8.2) | 116 (12.0) | 113 (11.7) | <0.001 |
| Peripheral vascular disease | 32 (3.3) | 41 (4.2) | 74 (7.7) | 110 (11.4) | <0.001 |
| Congestive heart failure | 33 (3.4) | 66 (6.8) | 99 (10.3) | 175 (18.1) | <0.001 |
| Kidney function | |||||
| Estimated GFR (ml/min per 1.73 m2) | 54.5±14.0 | 47.2±13.1 | 40.3±12.0 | 35.2±13.3 | <0.001 |
| Urine albumin-to-creatinine ratio (µg/mg)b | 15 (5, 121) | 31 (7, 268) | 104 (15, 716) | 212 (28, 1311) | <0.001 |
| Traditional CVD risk factors | |||||
| Current smoking | 75 (7.8) | 97 (10.0) | 132 (13.7) | 201 (20.8) | <0.001 |
| Body mass index (kg/m2) | 30.6±6.7 | 31.5±7.0 | 32.3±7.8 | 33.9±9.1 | <0.001 |
| Waist circumference (cm) | |||||
| Men | 103.0±14.7 | 106.5±15.3 | 107.6±16.0 | 110.4±17.1 | <0.001 |
| Women | 100.9±17.6 | 103.5±19.1 | 105.2±18.9 | 109.0±20.4 | <0.001 |
| Hemoglobin A1c (%)b | 5.8 (5.5, 6.5) | 6.1 (5.6, 7.2) | 6.3 (5.7, 7.6) | 6.5 (5.8, 7.9) | <0.001 |
| Total cholesterol (mg/dl) | 184±39 | 182±41 | 185±47 | 184±53 | 0.4 |
| LDL cholesterol (mg/dl) | 107±33 | 102±33 | 101±36 | 100±38 | <0.001 |
| HDL cholesterol (mg/dl) | 49±16 | 48±16 | 47±15 | 46±15 | <0.001 |
| Serum triglycerides (mg/dl)b | 112 (78, 160) | 128 (88, 183) | 131 (94, 194) | 140 (102, 207) | <0.001 |
| Nontraditional risk factors | |||||
| Hemoglobin (g/dl) | 13.5±1.6 | 12.8±1.7 | 12.4±1.6 | 11.8±1.8 | <0.001 |
| Serum albumin (mg/dl) | 4.1±0.4 | 4.0±0.4 | 3.9±0.5 | 3.8±0.5 | <0.001 |
| C-reactive protein (mg/L)b | 1.9 (0.9, 4.6) | 2.2 (1.0, 5.0) | 2.6 (1.1, 6.7) | 4.1 (1.5, 8.6) | <0.001 |
| Serum phosphate (mg/dl) | 3.4±0.5 | 3.6±0.6 | 3.8±0.6 | 4.1±0.8 | <0.001 |
| Serum calcium (mg/dl) | 9.2±0.4 | 9.2±0.5 | 9.2±0.5 | 9.1±0.6 | <0.001 |
| Intact PTH (pg/ml)b | 40 (29, 59) | 47 (33, 74) | 63 (40, 105) | 83 (47, 139) | <0.001 |
| N-terminal proBNP (pg/ml)b | 78 (35, 179) | 116 (54, 281) | 196 (82, 460) | 336 (136, 943) | <0.001 |
| Echocardiographyc | |||||
| Ejection fraction <50% | 129 (15.5) | 159 (18.9) | 185 (23.0) | 180 (24.2) | <0.001 |
| Left ventricular mass index (g/m2) | 47±12 | 50±13 | 53±13 | 58±15 | <0.001 |
| Medication use | |||||
| Antiplatelet | 392 (40.7) | 446 (46.2) | 461 (47.8) | 466 (48.3) | 0.01 |
| β-Blocker | 344 (35.7) | 446 (46.2) | 512 (53.1) | 588 (60.9) | <0.001 |
| ACE inhibitor/ARB | 577 (59.9) | 689 (71.3) | 702 (72.8) | 669 (69.3) | <0.001 |
| Statin | 431 (44.7) | 537 (55.6) | 589 (61.0) | 565 (58.6) | <0.001 |
| Loop diuretic | 175 (18.2) | 303 (31.4) | 405 (42.0) | 567 (58.8) | <0.001 |
Unless otherwise noted, values are n (%) or means ± SDs. CVD, cardiovascular disease; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker.
Sixteen percent of participants declined to respond.
Median (interquartile range).
Ejection fraction was available in 3225 participants and left ventricular mass index in 2880 participants.
Atherosclerotic Events
During a median follow-up of 3.6 years (interquartile range, 2.5–4.7 years), 287 participants experienced an atherosclerotic event that was adjudicated as possible, probable, or definite (22 total events/1000 person-years; 139 myocardial infarctions, 83 cerebrovascular accidents, 63 peripheral vascular disease procedures, 2 concurrent events). For the primary analysis, 490 participants were censored for onset of ESRD, and 228 for death. Among the 287 events, 116 incident events (40%) occurred in the 2681 participants without a history of atherosclerotic disease (12 incident events/1000 person-years).
In unadjusted Cox models, each doubling of FGF-23 levels was associated with a 37% increased risk of an atherosclerotic event, and ascending FGF-23 quartiles were associated with a stepwise increase in risk (Table 2). The hazard ratio (HR) comparing the highest versus the lowest quartile was 2.75 (95% confidence interval [CI], 2.19 to 3.46). Elevated FGF-23 remained significantly associated with greater risk of atherosclerotic events after sequential multivariable adjustment (Table 2). Each doubling of FGF-23 was associated with a 24% higher risk in the primary model that adjusted for demographic characteristics, kidney function, traditional cardiovascular risk factors, and cardiovascular medications (Figure 1A). In models that included other mineral metabolites and nontraditional risk factors, including inflammatory markers and hemoglobin, the highest versus lowest quartile of FGF-23 was associated with a 1.63-fold greater risk of atherosclerotic events (95% CI, 1.03 to 2.59), and a 23% higher risk per doubling of FGF-23. In these models, higher serum phosphate (HR, 1.09 per 0.5 mg/dl; 95% CI, 1.02 to 1.16) and calcium (HR, 1.09 per 0.5 mg/dl; 95% CI, 1.01 to 1.17) were also associated with atherosclerotic events, but parathyroid hormone (PTH) was not. The relationship between FGF-23 and atherosclerotic events modestly attenuated with additional adjustment for N-terminal pro–B-type natriuretic peptide (NT-proBNP; n=3317; HR, 1.19 per doubling; 95% CI, 1.01 to 1.40), but the point estimate was similar after additional adjustment for left ventricular mass index despite a reduction in power due to fewer participants with available measurements (n=2523; HR, 1.24 per doubling; 95% CI, 0.96 to 1.61).
Table 2.
Risk of first atherosclerotic and congestive heart failure events in the overall population by baseline levels of FGF-23
| Variable | FGF-23 | ||||
|---|---|---|---|---|---|
| <96.0 RU/ml | 96.0–145.4 RU/ml | 145.5–238.9 RU/ml | ≥239.0 RU/ml | Continuous per Doublinga | |
| Atherosclerotic events | |||||
| Events (n) | 47 | 62 | 78 | 100 | – |
| Incidence per 1000 person-years | 12.5 | 17.8 | 24.0 | 35.1 | – |
| HR (95% CI) | |||||
| Unadjustedb | Reference | 1.41 (1.08 to 1.85) | 1.90 (1.36 to 2.66) | 2.75 (2.19 to 3.46) | 1.37 (1.25 to 1.50) |
| Adjusted for demographic variables/kidney function c | Reference | 1.27 (0.97 to 1.66) | 1.54 (1.12 to 2.13) | 2.13 (1.68 to 2.69) | 1.30 (1.16 to 1.46) |
| Adjusted for traditional risk factors d | Reference | 1.32 (0.90 to 1.92) | 1.47 (1.14 to 1.90) | 1.76 (1.20 to 2.59) | 1.24 (1.09 to 1.40) |
| Adjusted for nontraditional risk factorse | Reference | 1.34 (0.90 to 1.98) | 1.46 (1.11 to 1.91) | 1.63 (1.03 to 2.59) | 1.23 (1.04 to 1.45) |
| Congestive heart failure hospitalization | |||||
| Events (n) | 30 | 52 | 96 | 182 | – |
| Incidence per 1000 person-years | 7.9 | 14.7 | 29.5 | 65.9 | – |
| HR (95% CI) | |||||
| Unadjustedb | Reference | 1.85 (1.12 to 3.05) | 3.69 (2.11 to 6.46) | 8.18 (5.47 to 12.24) | 1.75 (1.64 to 1.88) |
| Adjusted for demographic variables/kidney functionc | Reference | 1.45 (0.88 to 2.39) | 2.23 (1.23 to 4.03) | 4.40 (2.96 to 6.53) | 1.58 (1.51 to 1.65) |
| Adjusted for traditional risk factorsd | Reference | 1.36 (0.78 to 2.38) | 1.74 (1.06 to 2.86) | 2.98 (1.97 to 4.52) | 1.45 (1.28 to 1.65) |
| Adjusted for nontraditional risk factorse | Reference | 1.29 (0.69 to 2.40) | 1.57 (0.86 to 2.87) | 2.64 (1.59 to 4.39) | 1.39 (1.22 to 1.58) |
Risk per 1-unit change in log2(FGF-23).
All models are clustered by clinical center.
Adjusted for age, sex, race/ethnicity, income, eGFR, and urine albumin-to-creatinine ratio.
Adjusted for covariates in the demographic and kidney function adjusted model, plus history of hypertension; hypercholesterolemia; history of atherosclerotic cardiovascular disease; history of congestive heart failure; diabetes; control of BP to <140/90 mmHg; hemoglobin A1c; smoking status; body mass index; waist circumference; serum triglycerides; LDL cholesterol; use of antiplatelet medications, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, β-blockers, statins, and loop diuretics; and the number of prescribed classes of BP medications.
Adjusted for covariates in the traditional risk factor– and medication-adjusted model, plus serum phosphate, calcium, albumin, C-reactive protein, PTH, and hemoglobin.
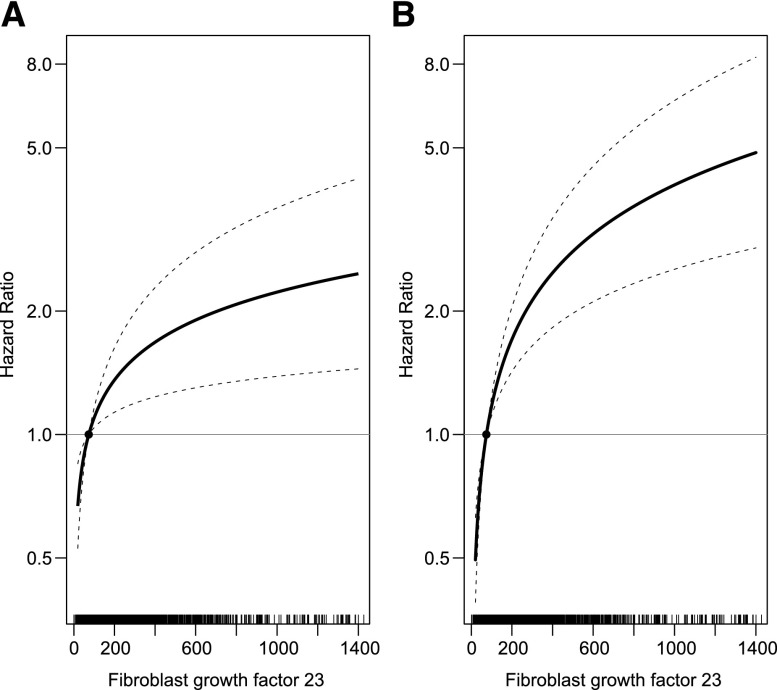
Figure 1.
FGF-23 is associated with atherosclerotic and congestive heart failure events. Multivariable-adjusted hazard ratios of cardiovascular events according to levels of FGF-23 on the arithmetic scale. (A) Atherosclerotic events. (B) Congestive heart failure events. Models are adjusted for age, sex, income, eGFR, urinary albumin-to-creatinine ratio; history of hypertension, hypercholesterolemia, atherosclerotic cardiovascular disease, congestive heart failure, and diabetes; control of BP <140/90 mmHg; hemoglobin A1c; smoking status; body mass index; waist circumference; serum triglycerides; LDL cholesterol; use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, β-blockers, statins, and loop diuretics; and the number of prescribed classes of BP medications. The median of the first quartile of FGF-23 (74 RU/ml) serves as the reference. Tick marks on the x axis represent individual participants’ FGF-23 levels.
Congestive Heart Failure Events
During a median follow-up of 3.7 years (interquartile range, 2.5–4.7 years), 360 participants were hospitalized for adjudicated congestive heart failure events that were classified as probable or definite (27 events/1000 person-years). For the primary analysis, 415 participants were censored for onset of ESRD and 215 for death. Among the 360 total events, 230 incident events (64%) occurred in the 3487 participants without a history of congestive heart failure (19 incident events/1000 person-years).
In unadjusted Cox models, each doubling of FGF-23 levels was associated with a 75% increased risk of congestive heart failure hospitalization, and ascending FGF-23 quartiles were associated with a stepwise increase in risk (Table 2). The HR comparing the highest versus the lowest quartile was 8.18 (95% CI, 5.47 to 12.24). The significant graded risk of congestive heart failure hospitalization across the spectrum of FGF-23 levels persisted in all multivariable models (Table 2). Each doubling of FGF-23 was associated with a 45% higher risk in the primary model that adjusted for demographic characteristics, kidney function, traditional cardiovascular risk factors, and cardiovascular medications (Figure 1B). In the model that included other mineral metabolites and nontraditional risk factors, the highest versus lowest quartile of FGF-23 was associated with a 2.64-fold greater risk of congestive heart failure (95% CI, 1.59 to 4.39), and a 39% higher risk per doubling of FGF-23. Higher serum phosphate was also associated with congestive heart failure (HR, 1.10 per 0.5 mg/dl; 95% CI, 1.01 to 1.19), but calcium and PTH were not. The relationship between FGF-23 and congestive heart failure events remained statistically significant after additional adjustment for NT-proBNP (n=3317; HR, 1.22 per doubling; 95% CI, 1.03 to 1.45) or left ventricular mass index (n=2523; HR, 1.27 per doubling; 95% CI, 1.08 to 1.49).
When dually analyzed in a single model that adjusted for demographic characteristics, kidney function, traditional cardiovascular risk factors, and medications, FGF-23 was significantly more strongly associated with congestive heart failure than with atherosclerotic events (P=0.02). The association of FGF-23 with cardiovascular events was qualitatively similar across strata of severity of kidney disease, proteinuria, prior cardiovascular disease, diabetes, BP control, anemia, 24-hour urinary sodium, income, baseline fat-free mass assessed by bioelectrical impedance as a surrogate of edema, left ventricular systolic function, and left ventricular hypertrophy (Figure 2).
Figure 2.
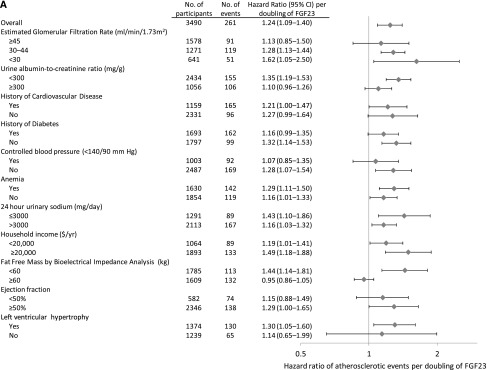
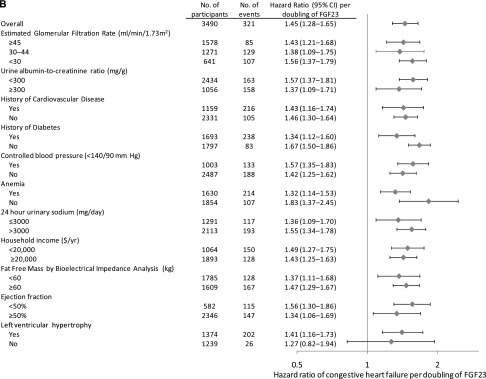
FGF-23 is associated with cardiovascular events across strata of cardiovascular risk factors. HRs ratio (diamonds) and 95% CIs (horizontal bars) of cardiovascular events by levels of FGF-23 in selected subgroups. (A) Atherosclerotic events. (B) Congestive heart failure events. Models are adjusted for age, sex, income, eGFR, urinary albumin-to-creatinine ratio; history of hypertension, hypercholesterolemia, atherosclerotic cardiovascular disease, congestive heart failure, and diabetes; control of BP<140/90 mmHg; hemoglobin A1c; smoking status; body mass index; waist circumference; serum triglycerides; LDL cholesterol; use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, β-blockers, statins, and loop diuretics; and the number of prescribed classes of BP medications. History of cardiovascular disease refers to a history of atherosclerotic or congestive heart failure. Anemia is defined as hemoglobin<12 g/dl in women and <13 g/dl in men. Total N does not sum across groups to 3860 because of missing data for individual covariates that were included in the multivariable models.
Incident Events
Point estimates for the association of FGF-23 with incident atherosclerotic events were similar to the analysis of total events but did not reach statistical significance in adjusted models (Table 3). In contrast, higher FGF-23 remained independently associated with incident congestive heart failure in all analyses (Table 3). The results were qualitatively unchanged when we introduced a 1-year lag between measurement of FGF-23 and assessment of outcomes to ensure that elevated FGF-23 preceded incident cardiovascular disease rather than occurred as a consequence of it: congestive heart failure—HR, 1.52 per doubling of FGF-23 (95% CI, 1.26 to 1.84); atherosclerotic events—HR, 1.26 (95% CI, 0.89 to 1.80).
Table 3.
Risk of incident atherosclerotic and congestive heart failure events among participants without a history of congestive heart failure (n=3487) or atherosclerotic disease (n=2681) by baseline levels of FGF-23
| Variable | FGF-23 | ||||
|---|---|---|---|---|---|
| <96.0 RU/ml | 96.0–145.4 RU/ml | 145.5–238.9 RU/ml | ≥239.0 RU/ml | Continuous per Doublinga | |
| Atherosclerotic events | |||||
| Events (n) | 25 | 22 | 32 | 37 | – |
| Incidence per 1000 person-years | 8.4 | 8.6 | 14.6 | 20.4 | – |
| HR (95% CI) | |||||
| Unadjustedb | Reference | 1.03 (0.64 to 1.66) | 1.73 (1.04 to 2.89) | 2.42 (1.76 to 3.34) | 1.34 (1.22 to 1.47) |
| Adjusted for demographic variables/kidney function c | Reference | 0.93 (0.53 to 1.62) | 1.39 (0.67 to 2.89) | 2.01 (1.12 to 3.63) | 1.27 (1.05 to 1.54) |
| Adjusted for traditional risk factors d | Reference | 0.92 (0.51 to 1.64) | 1.23 (0.63 to 2.41) | 1.66 (0.82 to 3.38) | 1.24 (0.96 to 1.60) |
| Adjusted for nontraditional risk factorse | Reference | 0.88 (0.47 to 1.65) | 1.26 (0.58 to 2.73) | 1.69 (0.68 to 4.18) | 1.25 (0.92 to 1.69) |
| Congestive heart failure hospitalization | |||||
| Events (n) | 25 | 31 | 70 | 104 | – |
| Incidence per 1000 person-years | 6.8 | 9.3 | 23.6 | 43.5 | – |
| HR (95% CI) | |||||
| Unadjustedb | Reference | 1.37 (0.85 to 2.21) | 3.48 (2.16 to 5.59) | 6.40 (4.37 to 9.38) | 1.74 (1.58 to 1.92) |
| Adjusted for demographic variables/kidney functionc | Reference | 1.01 (0.62 to 1.65) | 1.83 (1.00 to 3.33) | 2.94 (2.01 to 4.30) | 1.50 (1.36 to 1.66) |
| Adjusted for traditional risk factorsd | Reference | 1.09 (0.60 to 1.97) | 1.73 (1.04 to 2.86) | 2.36 (1.63 to 3.41) | 1.47 (1.27 to 1.69) |
| Adjusted for nontraditional risk factorse | Reference | 1.09 (0.63 to 1.87) | 1.66 (1.00 to 2.75) | 2.16 (1.49 to 3.13) | 1.41 (1.21 to 1.64) |
Risk per 1-unit change in log2(FGF-23).
All models are clustered by clinical center.
Adjusted for age, sex, race/ethnicity, income, eGFR, and urine albumin-to-creatinine ratio.
Adjusted for covariates in the demographic and kidney function adjusted model, plus history of hypertension; hypercholesterolemia; history of atherosclerotic cardiovascular disease; history of congestive heart failure; diabetes; control of BP to <140/90 mmHg; hemoglobin A1c; smoking status; body mass index; waist circumference; serum triglycerides; LDL cholesterol; use of antiplatelet medications, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, β-blockers, statins, and loop diuretics; and the number of prescribed classes of BP medications.
Adjusted for covariates in the traditional risk factor– and medication-adjusted model, plus serum phosphate, calcium, albumin, C-reactive protein, PTH, and hemoglobin.
Sensitivity Analyses
We performed a series of sensitivity analyses to verify the associations between FGF-23 and cardiovascular events (Table 4). To minimize the effect of potential misclassification of outcomes, we repeated the multivariable analyses restricted to definite events only. To account for out-of-hospital deaths that may have been due to cardiovascular causes, we reanalyzed each cardiovascular event subtype in a composite with death. To account for the possible influence that occurrence of one cardiovascular event type could have on the subsequent hazard of the other, we modeled history of atherosclerotic disease and congestive heart failure as time-varying covariates, updating these at the time of an incident event. Because missing data on individual covariates resulted in 10% loss of sample size in full models, we repeated the analysis after multiply imputing missing covariate data. The association between FGF-23 and congestive heart failure hospitalization was robust in all analyses. In contrast, analyses of “definite” atherosclerotic events were partially attenuated although CIs were also wider because of fewer events (Figure 3, Table 4). All associations were unchanged when we adjusted for 25-hydroxyvitamin D levels and for 125I-iothalamate GFR in place of eGFR (data not shown).
Table 4.
Adjusted HRs (95% CIs) of atherosclerotic event and congestive heart failure hospitalization by levels of FGF-23 in sensitivity analyses
| Model | Events (n)a | FGF-23 | ||||
|---|---|---|---|---|---|---|
| <96.0 RU/ml | 96.0–145.4 RU/ml | 145.5–238.9 RU/ml | ≥239.0 RU/ml | Continuous per Doublingb | ||
| Atherosclerotic event | ||||||
| Primary modelc | 261 | Reference | 1.32 (0.90 to 1.92) | 1.47 (1.14 to 1.90) | 1.76 (1.20 to 2.59) | 1.24 (1.09 to 1.40) |
| Definite event | 215 | Reference | 1.22 (0.89 to 1.66) | 1.31 (0.98 to 1.76) | 1.42 (0.90 to 2.23) | 1.19 (1.01 to 1.41) |
| Event or death | 468 | Reference | 1.28 (0.94 to 1.73) | 1.61 (1.34 to 1.93) | 2.36 (1.90 to 2.94) | 1.38 (1.30 to 1.46) |
| Including post-ESRD events | 306 | Reference | 1.41 (0.90 to 2.22) | 1.58 (1.22 to 2.04) | 1.89 (1.28 to 2.80) | 1.24 (1.15 to 1.34) |
| CHF as time-varying covariate | 261 | Reference | 1.31 (0.90 to 1.92) | 1.46 (1.13 to 1.89) | 1.74 (1.18 to 2.57) | 1.23 (1.08 to 1.39) |
| Multiple imputation of missing covariates | 287 | Reference | 1.12 (0.84 to 1.49) | 1.26 (0.96 to 1.64) | 1.59 (1.17 to 2.15) | 1.21 (1.07 to 1.36) |
| Congestive heart failure hospitalization | ||||||
| Primary modelc | 321 | Reference | 1.36 (0.78 to 2.38) | 1.74 (1.06 to 2.86) | 2.98 (1.97 to 4.52) | 1.45 (1.28 to 1.65) |
| Definite event | 235 | Reference | 1.40 (0.65 to 3.01) | 1.85 (0.93 to 3.67) | 3.30 (1.75 to 6.21) | 1.50 (1.29 to 1.74) |
| Event or death | 518 | Reference | 1.28 (1.07 to 1.53) | 1.70 (1.19 to 2.42) | 2.97 (2.59 to 3.40) | 1.49 (1.40 to 1.58) |
| Including post-ESRD events | 342 | Reference | 1.49 (0.87 to 2.56) | 1.83 (1.12 to 2.99) | 2.88 (1.80 to 4.62) | 1.40 (1.23 to 1.60) |
| ASCVD as time-varying covariate | 321 | Reference | 1.34 (0.76 to 2.36) | 1.72 (1.04 to 2.84) | 2.96 (1.95 to 4.48) | 1.45 (1.28 to 1.64) |
| Multiple imputation of missing covariates | 360 | Reference | 1.17 (0.68 to 2.00) | 1.65 (1.03 to 2.65) | 2.73 (1.89 to 3.95) | 1.43 (1.30 to 1.58) |
CHF, congestive heart failure; ASCVD, atherosclerotic cardiovascular disease.
The number of events represents events in adjusted models and may be lower than the overall number of events in the study population because of model-wise deletion.
Risk per 1-unit change in log2(FGF-23).
Adjusted for age; sex; race/ethnicity; household income; eGFR; urine albumin-to-creatinine ratio; history of hypertension; hypercholesterolemia; history of atherosclerotic cardiovascular disease; history of congestive heart failure; diabetes; control of BP to <140/90 mmHg; hemoglobin A1c; smoking status; body mass index; waist circumference; serumtriglycerides; LDL cholesterol; use of antiplateletmedications, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, β-blockers, statins, and loop diuretics; and the number of classes of BP medications used. Clustered by center.
Figure 3.
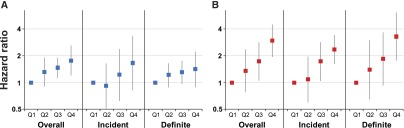
FGF-23 is more strongly associated with congestive heart failure compared to atherosclerotic events in overall, incident and definite event analyses. HRs (squares) and 95% CIs (vertical bars) for overall, incident, and definite atherosclerotic (A) and congestive heart failure events (B) by quartiles (Q) of FGF-23. Models are adjusted for demographic variables, kidney function, traditional cardiovascular risk factors, and medications. Q1 served as the reference group for all analyses.
Discussion
Cardiovascular disease is the leading cause of death in patients with CKD,26 and elevated FGF-23 is a powerful predictor of mortality.16,18,19 In the current prospective cohort study, we demonstrate that elevated FGF-23 is independently associated with occurrence of cardiovascular disease events in CKD stages 2–4 and that elevated FGF-23 is more strongly associated with risk of congestive heart failure than atherosclerotic events.
The strong association between FGF-23 and congestive heart failure may be a clinical consequence of the direct hypertrophic effects of FGF-23 on the myocardium,20 but other possibilities could also contribute to our results. Because FGF-23 correlates with GFR, it is possible that unmeasured confounding related to severity of CKD could mediate the association. However, our results were unchanged when we stratified across or adjusted for a comprehensive set of covariates, including directly measured GFR. Furthermore, other CKD-specific factors that correlated with GFR to a similar extent in the CRIC study, such as PTH,18 were not associated with risk of congestive heart failure. Residual confounding by cardiovascular risk factors that are associated with baseline FGF-23 levels is another possibility.27 However, our results were robust in subgroups with favorable risk factor profiles, including those with no history of heart disease, diabetes, or anemia, and those with well controlled BP, higher income, and relatively low 24-hour urine sodium. Finally, it is interesting to note that the association between FGF-23 and congestive heart failure remained significant after adjustment for left ventricular mass index and that the effect size was similar in the subgroup of patients without left ventricular hypertrophy. These results suggest that changes in left ventricular mass and geometry may not fully mediate the strong association between FGF-23 and congestive heart failure.
The etiology of congestive heart failure events is particularly complex in patients with CKD, who are prone to volume overload because of reduced GFR or nephrotic syndrome, and in patients with ESRD in whom an inadequate dialysis prescription or poor adherence may contribute. Nevertheless, the association between elevated FGF-23 and greater risk of congestive heart failure events was similar in patients with earlier or later stages of CKD and among those with substantial or minimal proteinuria, and it was similarly robust regardless of whether we included or excluded events that occurred after onset of ESRD. Furthermore, congestive heart failure hospitalizations often represent exacerbations of an indolent disease process, such that the exact time of onset can be difficult to pinpoint. This natural history introduces the possibility of a “reverse causal” process in which elevated FGF-23 could reflect an early consequence rather than an upstream cause of heart failure.28 This scenario is also unlikely to explain our results because patients with a history of New York Heart Association class 3–4 heart failure were excluded from the CRIC study and our results were equally strong in lag analyses that exclusively considered incident heart failure events that occurred at least 1 year after FGF-23 was measured. Although our primary goal was to study novel disease mechanisms rather than to determine the predictive utility of FGF-23 as a biomarker of future heart failure risk, its independent association with congestive heart failure even after adjustment for NT-proBNP levels suggests that future studies should investigate FGF-23 in a panel of risk prediction biomarkers for congestive heart failure events.
Previous community-based studies similarly reported that higher levels of FGF-23 were associated with left ventricular hypertrophy, reduced ejection fraction, congestive heart failure events, and cardiovascular mortality.17,29–31 Furthermore, many of these associations were more pronounced among participants with CKD than in those without.17,29,30 The similarly strong association reported in this study of a large CKD population supports the notion that FGF-23 may be a particularly relevant risk factor for cardiovascular disease in patients with CKD. Because presence of CKD raises FGF-23 levels, it is possible that the stronger association of FGF-23 with cardiovascular disease in CKD reflects the effect of exposure to higher FGF-23 levels in this population, but additional mechanisms may contribute. For example, CKD is characterized by decreased renal expression of Klotho, which is the co-receptor that enhances the binding affinity of FGF-23 for FGF receptors in the kidney, where it exerts its classic effect on mineral metabolism. In contrast, the pro-hypertrophic effects of FGF-23 on the myocardium are FGF receptor dependent but occur in the absence of Klotho, which is not expressed by cardiac myocytes.20 Thus, the adverse effects of elevated FGF-23 on the heart may be exaggerated in CKD because of the combination of high FGF-23 levels and Klotho deficiency, which frees FGF-23 to increase binding to extrarenal FGF receptors, such as in the heart.
In contrast to the analyses of congestive heart failure, the association between FGF-23 and atherosclerotic events was weaker. Although prior studies reported minimal diurnal variation in FGF-2332 and highly stable FGF-23 levels over years in patients with stable kidney function,14 use of single baseline measurements of FGF-23 may have biased these findings toward the null. However, prior reports of the association between FGF-23 and atherosclerotic events were also conflicting.16,17,33–36 Similar to the current study, elevated FGF-23 was not associated with incident atherosclerotic events in the Health Professionals Follow-up Study,33 and previous studies that reported a significant association could not comprehensively adjust for baseline atherosclerotic risk factors that are associated with elevated FGF-23.35,36 Thus, the less robust association between atherosclerotic disease and FGF-23 in this study could represent residual confounding by incomplete ascertainment of the severity or duration of exposure to traditional atherosclerotic risk factors. Alternatively, the lower number of atherosclerotic events combined with our inability to ascertain out-of-hospital sudden cardiac death may have reduced the power of these analyses to detect a true association between FGF-23 and atherosclerotic events. In the absence of direct effects on the arterial vasculature, elevated FGF-23 levels could plausibly relate to atherosclerotic events indirectly through its associations with inflammation or Klotho deficiency, which promotes arterial calcification.25,37,38
Interestingly, the association between FGF-23 and atherosclerotic events attenuated somewhat when we restricted the analysis to definite events. Although this could be due to fewer total events with a reduction in power, a less robust biologic relationship between FGF-23 and atherosclerotic events is also possible. According to the adjudication criteria, modestly elevated troponin levels without diagnostic electrocardiographic changes could have been classified as a possible or probable, but not a definite, myocardial infarction. Recent studies demonstrate that acute decompensated heart failure often manifests elevated cardiac troponins,39 possibly as a consequence of increased myocardial wall stress, and that low-grade troponin elevation strongly predicts incident heart failure.40,41 Low-grade troponin elevation is especially common among ambulatory patients with CKD, in whom it is also associated with left ventricular hypertrophy and elevated FGF-23 levels.42,43 The weaker association of FGF-23 with definite atherosclerotic events could have resulted from misclassification of low-grade troponin elevations as possible or probable atherosclerotic events when they were actually due to cardiac remodeling. Thus, certain participants’ low-grade troponin elevations may have been an early manifestation of their predisposition to congestive heart failure rather than coronary artery disease events.
The excess burden of congestive heart failure in CKD has been assumed to result from the high prevalence of atherosclerotic disease risk factors, anemia, and left ventricular pressure and volume overload due to hypertension and impaired sodium excretion that accompanies reduced GFR. In our study, FGF-23 was strongly associated with congestive heart failure independent of both estimated and measured GFR, and even among those without traditional and CKD-specific risk factors for cardiovascular disease, or those in whom these risk factors were well controlled. These findings suggest that FGF-23 could represent a novel mechanism of congestive heart failure that mediates at least a portion of excess cardiovascular disease risk attributable to CKD. Interventional studies are needed to determine whether reducing FGF-23 levels will prevent cardiovascular events in patients with CKD.
Concise Methods
Study Design and Population
The CRIC study is a racially and ethnically diverse, multicenter prospective cohort study that aims to identify risk factors for cardiovascular disease and progression of CKD.44 The CRIC study enrolled 3939 adults aged 21–74 years with CKD stages 2–4 at 13 centers in the United States between 2003 and 2008. At enrollment, participants had an eGFR of 20–70 ml/min per 1.73 m2. Major exclusion criteria included institutionalization, inability to provide informed consent, pregnancy, polycystic kidney disease, previous treatment with dialysis for >1 month, and New York Heart Association class 3–4 heart failure.45 Participants underwent annual study visits and biannual follow-up by telephone. The final study population for this analysis consisted of 3860 participants after exclusion of 79 with inadequate plasma samples for FGF-23 measurement or lack of follow-up for cardiovascular events. The protocol was approved by the human research committees at each participating center, and all participants provided written informed consent.
Data Collection
The CRIC study’s central laboratory measured baseline plasma C-terminal FGF-23 (Immutopics, San Clemente, CA) in duplicate with intraassay coefficient of variation <10%. Demographic and clinical information were obtained at the baseline visit by questionnaires, interviews, and physical examination. Diabetes was defined by a fasting glucose≥126 mg/dl or use of insulin or oral hypoglycemic medications; hypertension by a systolic BP≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive medications; and hypercholesterolemia by a total serum cholesterol>200 mg/dl or use of cholesterol-lowering medications. History of cardiovascular disease was established by a self-reported history of congestive heart failure, myocardial infarction, coronary revascularization, cerebrovascular accident, peripheral artery revascularization, or amputation. Baseline laboratory covariates, including serum phosphate, calcium, albumin, total intact PTH (Scantibodies, Santee, CA), hemoglobin, high-sensitivity C-reactive protein, plasma NT-proBNP (Roche Diagnostics, Indianapolis, IN), and fasting lipid measures, were determined in a central laboratory. GFR was estimated from serum creatinine using the CKD-Epidemiology Collaboration equation46 and was directly measured as the clearance of 125I-iothalamate in a subset of 1414 participants. Body composition was assessed by body mass index, waist circumference, and bioelectrical impedance. Two-dimensional transthoracic echocardiography was performed within 2 years of study entry in 3323 participants (86%) at a median of 378 days from study baseline (interquartile range, 344–419 days). Left ventricular mass was indexed to height2.7, and ejection fraction was measured as previously described.20 Left ventricular hypertrophy was defined as a left ventricular mass index≥50 g/m2.7 in men or ≥47 g/m2.7 in women.
Outcomes
The two primary adjudicated outcomes were first hospitalization for congestive heart failure and first atherosclerotic event, including hospitalization for myocardial infarction, cerebrovascular accident, or peripheral vascular disease, which occurred between enrollment and June 30, 2009. In addition to censoring for death, we censored our primary analyses for onset of ESRD because of difficulty in distinguishing congestive heart failure from volume overload and potential differences in the pathogenesis of cardiovascular events among patients undergoing dialysis.
Adjudication
Hospitalizations were self-reported by participants every 6 months by telephone or at in-person follow-up visits. Study personnel identified possible cardiovascular events by reviewing hospital billing codes. Two independent reviewers adjudicated cardiovascular events using hospital records and classified them as probable or definite, except for myocardial infarction, which was also classified as possible. Criteria for congestive heart failure events included a combination of symptoms (dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea) accompanied by consistent findings on chest radiography (pulmonary edema, congestion) or physical examination (documentation of two or more of the following: pulmonary rales, S3 gallop, jugular venous distention >5 cm, peripheral edema). Criteria for myocardial infarction included a combination of chest pain, electrocardiography abnormalities, and elevated cardiac biomarkers. Peripheral vascular disease procedures were ascertained using International Classification of Diseases, Ninth Revision, codes. Two neurologists adjudicated cerebrovascular accidents. Further details of event adjudication are provided in the Supplemental Material.
Statistical Analyses
We calculated incidence rates of atherosclerotic and congestive heart failure events by FGF-23 levels modeled as a log-transformed continuous variable and in quartiles. Using Cox models, we separately evaluated time to each event type in the full study population and incident events in the population without a history of congestive heart failure (n=3487) or atherosclerotic disease (n=2681). Because initial analyses demonstrated linear relationships between log FGF-23 and log hazard of events, risks were reported on a linear continuous scale per unit of doubling of FGF-23. To further aid in interpretation of the results, FGF-23 was also analyzed in quartiles as we and others have done previously.14,18 To minimize the possibility that an ongoing subclinical cardiovascular disease process at baseline could have raised FGF-23 levels and hence inflated estimates of risk attributable to baseline FGF-23, we performed additional analyses that exclusively considered incident events that occurred ≥1 year after FGF-23 was measured.
We adjusted Cox models sequentially for demographic variables (age, sex, race, ethnicity, and income), kidney function (eGFR and urinary albumin-to-creatinine ratio), traditional cardiovascular risk factors (history of hypertension, hypercholesterolemia, atherosclerotic disease, congestive heart failure, and diabetes; control of BP<140/90 mmHg; hemoglobin A1c; smoking [never, former, or current]; body mass index; waist circumference; serum triglycerides; LDL cholesterol), use of cardiovascular medications (antiplatelet agents, angiotensin-receptor blockers or angiotensin-converting enzyme inhibitors, β-blockers, statins, loop diuretics), and the number of prescribed classes of antihypertensive drugs (≤1, 2, 3, or ≥4). We further adjusted for nontraditional risk factors that have been linked to cardiovascular disease in CKD populations, including serum phosphate, calcium, albumin, C-reactive protein, PTH, and hemoglobin. Secondary analyses separately adjusted for NT-proBNP, a biomarker of myocardial pressure and volume overload, and left ventricular mass index, a factor that may mediate an association between FGF-23 and cardiovascular events.20 All analyses were clustered by study center to correct standard errors for correlation structure. Because adjustment or stratification for study center did not qualitatively change results, these were not included in the final models.
To test whether elevated FGF-23 was more strongly associated with congestive heart failure than with atherosclerotic events, we included both outcomes in a single model allowing distinct baseline hazard functions and distinct covariate effects on each outcome. We compared the effects of FGF-23 on cause-specific hazards of each outcome in this model using the Wald chi-square test. The proportional hazards assumption was confirmed for all models using Schoenfeld residuals and log-log plots.
Subgroup Analyses
We investigated the association between FGF-23 and cardiovascular events in analyses stratified by covariates that may contribute to cardiovascular disease, including levels of kidney function (eGFR ≥45, 30–44 and <30 ml/min per 1.73 m2) and proteinuria (urinary albumin-to-creatinine ratio <300 mg/g versus ≥300 mg/g), history of cardiovascular disease, diabetes, BP control (<140/90 mmHg), anemia (hemoglobin <13 g/dl in men and <12 g/dl in women), 24-hour urine sodium (≤3000 mg/d versus >3000 mg/d), income (<$20,000/year versus ≥$20,000/year), categories of fat-free mass determined by bioelectrical impedance analysis as a surrogate of edema (<60 kg versus ≥60 kg), left ventricular ejection fraction (<50% versus ≥50%), and presence of left ventricular hypertrophy.
Sensitivity Analyses
We performed the following sensitivity analyses: (1) analyses restricted to events meeting more stringent adjudication criteria and classified as “definite”; (2) analyses of the composite outcome of each cardiovascular event type with death; (3) analyses that included events that occurred after onset of ESRD; (4) analyses that incorporated atherosclerotic or congestive heart failure events that occurred after study enrollment as time-dependent covariates such that medical history was updated at the time of a new diagnosis; and (5) analyses in which missing covariates were multiply imputed using chained equations (n=5 cycles).47 In additional sensitivity analyses, we adjusted for 125I-iothalamate GFR in lieu of eGFR and for 25-hydroxyvitamin D levels in the subset of participants with these results (n=1369). Analyses were performed using Stata 11.1 (Stata Corp., College Station, TX), SAS 9.2 (SAS Institute, Inc., Cary, NC), and R, version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).
Disclosures
T.I. has served as a consultant and received honoraria from Shire and Genzyme. M.W. has served as a consultant for or received honoraria from Abbott Laboratories, Amgen, Genzyme, Kai, and Lutipold.
Supplementary Material
Acknowledgments
Funding for the CRIC study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the University of Pennsylvania CTRC CTSA UL1 RR-024134, Johns Hopkins University UL1 RR-025005, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1RR024986, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, and Kaiser NIH/NCRR UCSF-CTSI UL1 RR-024131. J.J.S. was supported by grant K23DK095949; A.H.A. by K01DK092353; D.S.R. by R01DK073665, HL268200900040C, and R01HL107241; and M.W. by R01DK076116, R01DK081374, R01DK094796, K24DK093723, and U54TR000255, all from the NIH.
CRIC Study Investigators additionally include Lawrence J. Appel and Raymond R. Townsend
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050465/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, James MT, Hemmelgarn BR, Alberta Kidney Disease Network : Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 380: 807–814, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J: Reduced kidney function as a risk factor for incident heart failure: The atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol 18: 1307–1315, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Dhingra R, Gaziano JM, Djoussé L: Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail 4: 138–144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A: The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens 13: 163–170, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Middleton RJ, Parfrey PS, Foley RN: Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol 12: 1079–1084, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG: Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 113: 2713–2723, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, Randall O, Rostand S, Sherer S, Toto RD, Wright JT, Jr, Wang X, Greene T, Appel LJ, Lewis J, AASK Study Group : Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 48: 739–751, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Wolf M: Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 82: 737–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scialla J, Astor B, Isakova T, Xie H, Appel L, Wolf M: Mineral metabolites and chronic kidney disease progression in African Americans. J Am Soc Nephrol 24: 125–135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG: Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60: 200–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeherunvong W, Abitbol CL, Chandar J, Rusconi P, Zilleruelo GE, Freundlich M: Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol 27: 2129–2136, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Mizobuchi M, Towler D, Slatopolsky E: Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol 20: 1453–1464, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Scialla J, Lau W, Reilly M, Isakova T, Yang H, Crouthamel M, Chavkin N, Rahman M, Wahl P, Amaral A, Hamano T, Master S, Nessel L, Chai B, Xie D, Kallem R, Chen J, Lash J, Kusek J, Budoff M, Giachelli C, Wolf M; Chronic Renal Insufficiency Cohort Study Investigators. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int 83: 1159–1168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim K, Lu T-S, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao L-L: Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 125: 2243–2255, 2012 [DOI] [PubMed] [Google Scholar]
- 26.United States Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 27.Gutiérrez OM, Wolf M, Taylor EN: Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin J Am Soc Nephrol 6: 2871–2878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plischke M, Neuhold S, Adlbrecht C, Bielesz B, Shayganfar S, Bieglmayer C, Szekeres T, Hörl WH, Strunk G, Vavken P, Pacher R, Hülsmann M: Inorganic phosphate and FGF-23 predict outcome in stable systolic heart failure. Eur J Clin Invest 42: 649–656, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE: Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 207: 546–551, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, Larsson TE: Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int 83: 160–166, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Seiler S, Cremers B, Rebling NM, Hornof F, Jeken J, Kersting S, Steimle C, Ege P, Fehrenz M, Rogacev KS, Scheller B, Böhm M, Fliser D, Heine GH: The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur Heart J 32: 2688–2696, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Isakova T, Gutierrez O, Shah A, Castaldo L, Holmes J, Lee H, Wolf M: Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol 19: 615–623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor EN, Rimm EB, Stampfer MJ, Curhan GC: Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J 161: 956–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seiler S, Reichart B, Roth D, Seibert E, Fliser D, Heine GH: FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant 25: 3983–3989, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Nakano C, Hamano T, Fujii N, Obi Y, Matsui I, Tomida K, Mikami S, Inoue K, Shimomura A, Nagasawa Y, Okada N, Tsubakihara Y, Rakugi H, Isaka Y: Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 50: 1266–1274, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M, Chronic Renal Insufficiency Cohort : Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 7: 1155–1162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peacock WF, 4th, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH, ADHERE Investigators : Cardiac troponin and outcome in acute heart failure. N Engl J Med 358: 2117–2126, 2008 [DOI] [PubMed] [Google Scholar]
- 40.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK: Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 304: 2503–2512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM: Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 123: 1367–1376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith K, deFilippi C, Isakova T, Gutiérrez OM, Laliberte K, Seliger S, Kelley W, Duh SH, Hise M, Christenson R, Wolf M, Januzzi J: Fibroblast growth factor 23, high-sensitivity cardiac troponin, and left ventricular hypertrophy in CKD. Am J Kidney Dis 61: 67–73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, Wians F, Sabatine MS, Morrow DA, de Lemos JA: Prevalence and determinants of troponin T elevation in the general population. Circulation 113: 1958–1965, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Royston P: Multiple imputation of missing values: Update of ice. Stata J 5: 527–536, 2005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.