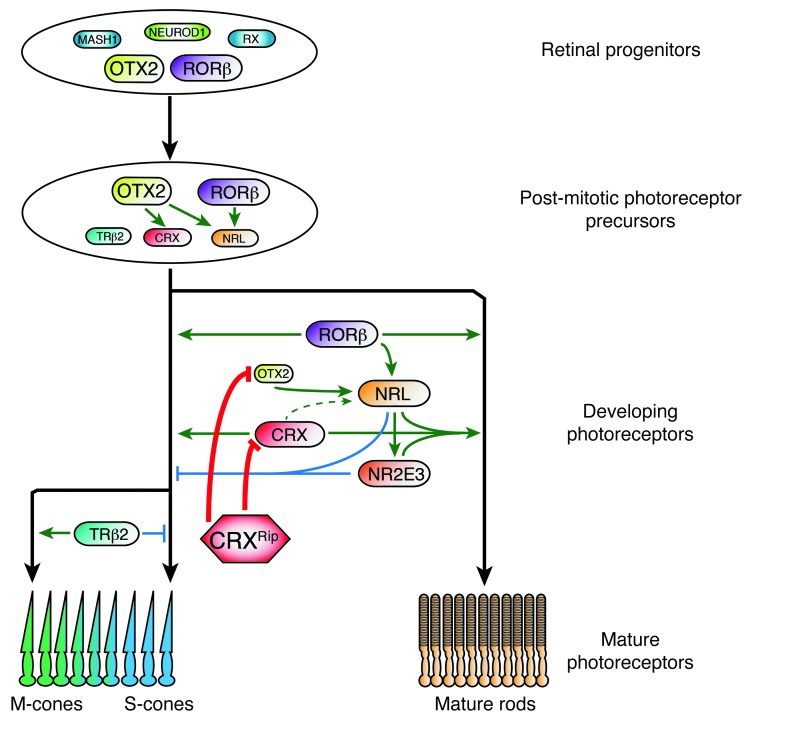
Figure 9. The molecular mechanism of congenital blindness caused by dominant CRX frameshift mutations.
After cell cycle exit, and under the control of OTX2, postmitotic precursors get restricted to the photoreceptor lineage and are fated to produce both rods and cones. These precursors will differentiate by default into S-cones, unless their fate is directed into rods by the expression of NRL, or into M-cones by the expression of TRβ2. In developing photoreceptors, NRL expression is initiated by OTX2 and RORβ and increases during development to restrict the lineage to rods. OTX2 plays a crucial role in maintaining NRL expression to consolidate rod cell fate. Sustained expression of NRL is needed to induce downstream targets, including NR2E3, that are critical for suppressing cone genes and for rod maturation in collaboration with CRX. In CrxRip/+ mutant retinas, CRXRip protein blocks both OTX2 and CRX, arresting NRL expression and, consequently, rod differentiation pathway. In addition, CRXRip protein prevents the CRXWT protein from forming requisite transcriptional complexes for rod gene expression. Ultimately, the arrest in photoreceptor development does not permit phototransduction, causing congenital blindness.