Abstract
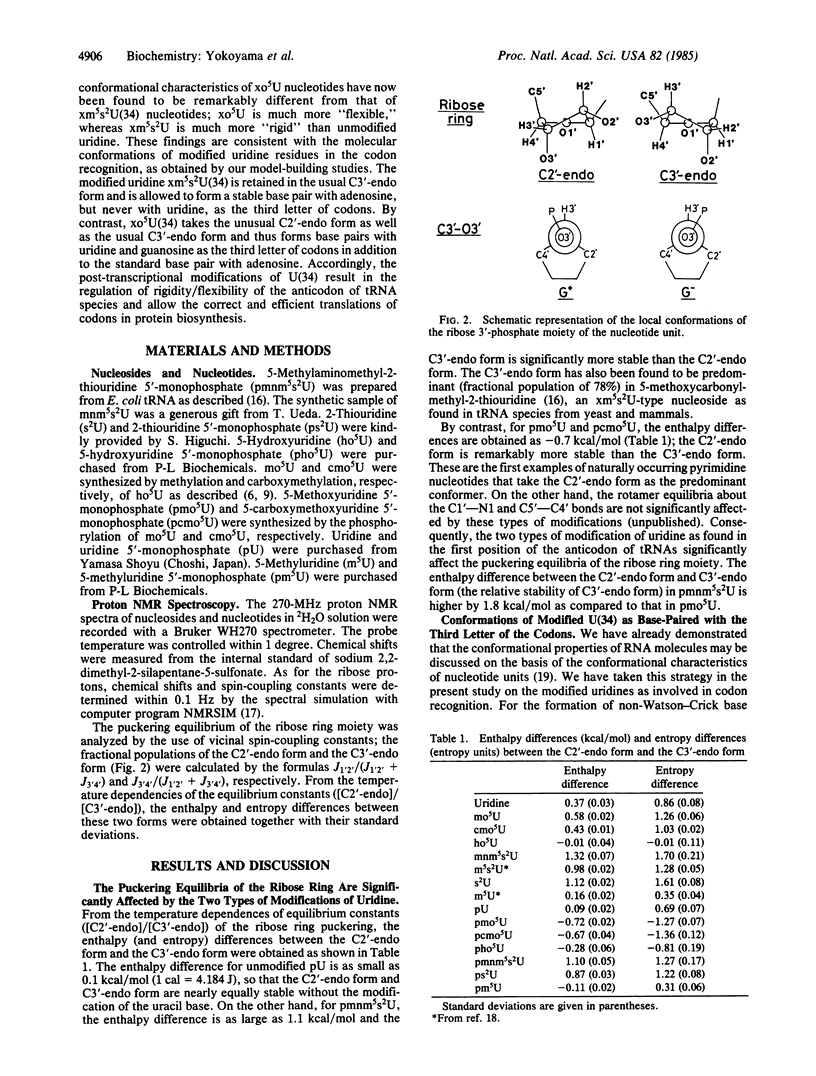
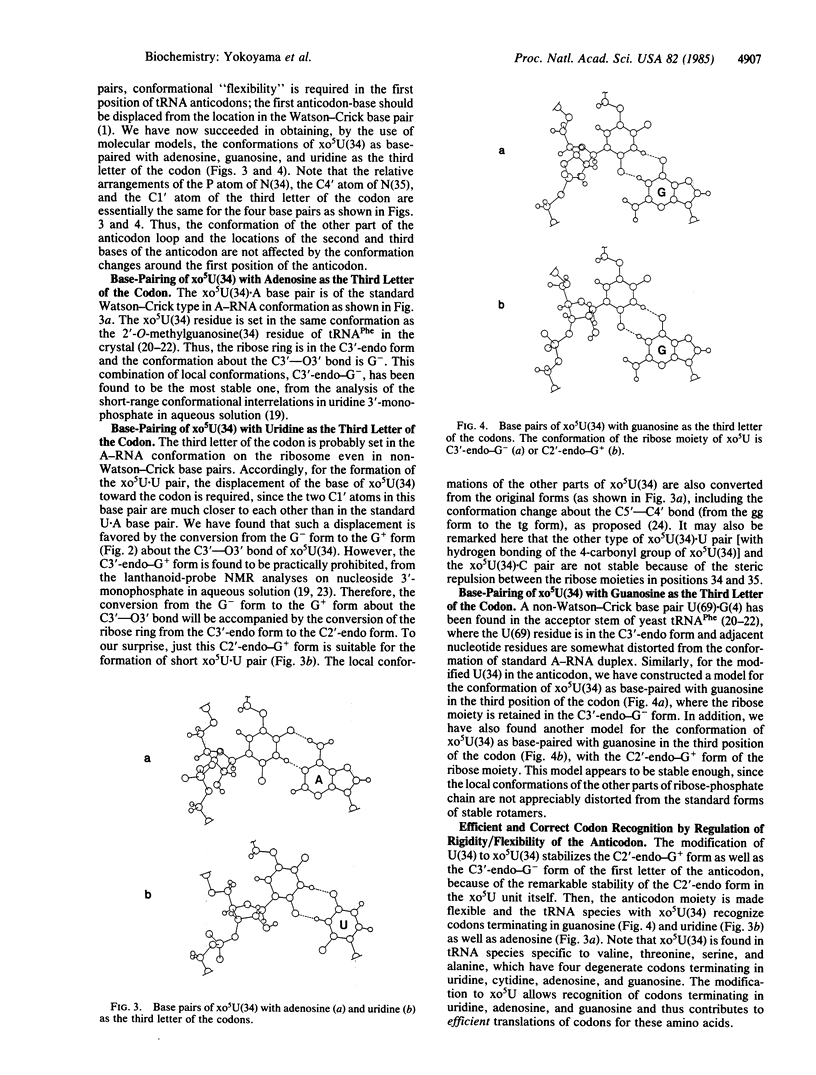
Proton NMR analyses have been made to elucidate the conformational characteristics of modified nucleotides as found in the first position of the anticodon of tRNA [derivatives of 5-methyl-2-thiouridine 5'-monophosphate (pxm5s2U) and derivatives of 5-hydroxyuridine 5'-monophosphate (pxo5U)]. In pxm5s2U, the C3'-endo form is extraordinarily more stable than the C2'-endo form for the ribose ring, because of the combined effects of the 2-thiocarbonyl group and the 5-substituent. By contrast, in pxo5U, the C2'-endo form is much more stable than the C3'-endo form, because of the interaction between the 5-substituent and the 5'-phosphate group. The enthalpy differences between the C2'-endo form and the C3'-endo form have been obtained as 1.1, -0.7, and 0.1 kcal/mol (1 cal = 4.184 J) for pxm5s2U, pxo5U, and unmodified uridine 5'-monophosphate, respectively. These findings lead to the conclusion that xm5s2U in the first position of the anticodon exclusively takes the C3'-endo form to recognize adenosine (but not uridine) as the third letter of the codon, whereas xo5U takes the C2'-endo form as well as the C3'-endo form to recognize adenosine, guanosine, and uridine as the third letter of the codon on ribosome. Accordingly, the biological significance of such modifications of uridine to xm5s2U/xo5U is in the regulation of the conformational rigidity/flexibility in the first position of the anticodon so as to guarantee the correct and efficient translation of codons in protein biosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Grosjean H. J., de Henau S., Crothers D. M. On the physical basis for ambiguity in genetic coding interactions. Proc Natl Acad Sci U S A. 1978 Feb;75(2):610–614. doi: 10.1073/pnas.75.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Kimura F., Nishimura S. Nucleotide sequence of valine tRNA 1 from Escherichia coli B. Biochim Biophys Acta. 1969 Dec 16;195(2):590–592. doi: 10.1016/0005-2787(69)90671-6. [DOI] [PubMed] [Google Scholar]
- Hillen W., Egert E., Lindner H. J., Gassen H. G. Crystal and molecular structure of 2-thio-5 carboxymethyluridine and its methyl ester: helix terminator nucleosides in the first position of some anticodons. Biochemistry. 1978 Nov 28;17(24):5314–5320. doi: 10.1021/bi00617a036. [DOI] [PubMed] [Google Scholar]
- Hillen W., Egert E., Lindner H. J., Gassen H. G. Restriction or amplification of wobble recognition: the structure of 2-thio-5-methylaminomethyluridine and the interaction of odd uridines with the anticodon loop backbone. FEBS Lett. 1978 Oct 15;94(2):361–364. doi: 10.1016/0014-5793(78)80977-6. [DOI] [PubMed] [Google Scholar]
- Ishikura H., Yamada Y., Nishimura S. Structure of serine tRNA from Escherichia coli. I. Purification of serine tRNA's with different codon responses. Biochim Biophys Acta. 1971 Jan 28;228(2):471–481. doi: 10.1016/0005-2787(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S., Vorbrüggen H., Iitaka Y. Crystal and molecular structure of the acetonide of 5-methylaminomethyl-2-thiouridine: a minor constituent of Escherichia coli tRNAs. FEBS Lett. 1979 Jul 15;103(2):270–273. doi: 10.1016/0014-5793(79)81343-5. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Kimura F., Harada F., Nishimura S. Primary sequence of tRNA-Val-1 from Escherichia coli B. II. Isolation of large fragments by limited digestion with RNases, and overlapping of fragments to reduce the total primary sequence. Biochemistry. 1971 Aug 17;10(17):3277–3283. doi: 10.1021/bi00793a018. [DOI] [PubMed] [Google Scholar]
- Lustig F., Elias P., Axberg T., Samuelsson T., Tittawella I., Lagerkvist U. Codon reading and translational error. Reading of the glutamine and lysine codons during protein synthesis in vitro. J Biol Chem. 1981 Mar 25;256(6):2635–2643. [PubMed] [Google Scholar]
- Mitra S. K., Lustig F., Akesson B., Axberg T., Elias P., Lagerkvist U. Relative efficiency of anticodons in reading the valine codons during protein synthesis in vitro. J Biol Chem. 1979 Jul 25;254(14):6397–6401. [PubMed] [Google Scholar]
- Morikawa K., Torii K., Iitaka Y., Tsuboi M., Nishimura S. Crystal and molecular structure of the methyl ester of uridin-5-oxyacetic acid: a minor constituent of Escherichia coli tRNAs. FEBS Lett. 1974 Nov 15;48(2):279–282. doi: 10.1016/0014-5793(74)80486-2. [DOI] [PubMed] [Google Scholar]
- Murao K., Hasegawa T., Ishikura H. 5-methoxyuridine: a new minor constituent located in the first position of the anticodon of tRNAAla, tRNAThr, and tRNAVal from Bacillus subtilis. Nucleic Acids Res. 1976 Oct;3(10):2851–2860. doi: 10.1093/nar/3.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao K., Hasegawa T., Ishikura H. Nucleotide sequence of valine tRNA mo5UAC from bacillus subtilis. Nucleic Acids Res. 1982 Jan 22;10(2):715–718. doi: 10.1093/nar/10.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao K., Saneyoshi M., Harada F., Nishimura S. Uridin-5-oxy acetic acid: a new minor constituent from E. coli valine transfer RNA I. Biochem Biophys Res Commun. 1970 Feb 20;38(4):657–662. doi: 10.1016/0006-291x(70)90631-5. [DOI] [PubMed] [Google Scholar]
- Oda K., Kimura F., Harada F., Nishimura S. Restoration of valine acceptor activity by combining oligonucleotide fragments derived from a Bacillus subtilis ribonuclease digest of Escherichia coli valine transfer RNA. Biochim Biophys Acta. 1969 Mar 18;179(1):97–105. doi: 10.1016/0005-2787(69)90125-7. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Seeman N. C., Wang A. H., Suddath F. L., Rich A. Yeast phenylalanine transfer RNA: atomic coordinates and torsion angles. Nucleic Acids Res. 1975 Dec;2(12):2329–2341. doi: 10.1093/nar/2.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertus J. D., Ladner J. E., Finch J. T., Rhodes D., Brown R. S., Clark B. F., Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974 Aug 16;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Axberg T., Fölsch G., Akesson B., Lagerkvist U. Aberrations of the classic codon reading scheme during protein synthesis in vitro. J Biol Chem. 1980 May 25;255(10):4583–4588. [PubMed] [Google Scholar]
- Sekiya T., Takeishi K., Ukita T. Specificity of yeast glutamic acid transfer RNA for codon recognition. Biochim Biophys Acta. 1969 Jun 17;182(2):411–426. doi: 10.1016/0005-2787(69)90192-0. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Takemoto T., Nishimura S., Ukita T. Selective utilization of valyl-tRNA having a particular coding specificity in a rabbit hemoglobin synthesizing system. Biochem Biophys Res Commun. 1972 May 26;47(4):746–752. doi: 10.1016/0006-291x(72)90555-4. [DOI] [PubMed] [Google Scholar]
- Takemoto T., Takeishi K., Nishimura S., Ukita T. Transfer of valine into rabbit haemoglobin from various isoaccepting species of valyl-tRNA differing in codon recognition. Eur J Biochem. 1973 Oct 18;38(3):489–496. doi: 10.1111/j.1432-1033.1973.tb03084.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Yokoyama S., Hansske F., Kasai H., Miyazawa T. CD and NMR studies on the conformational thermostability of 2-thioribothymidine found in the T psi C loop of thermophile tRNA. Biochem Biophys Res Commun. 1979 Nov 28;91(2):671–677. doi: 10.1016/0006-291x(79)91574-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Yokoyama S., Miyazawa T., Watanabe K., Higuchi S. NMR analyses on the molecular mechanism of the conformational rigidity of 2-thioribothymidine, a modified nucleoside in extreme thermophile tRNAs. FEBS Lett. 1983 Jun 27;157(1):95–99. doi: 10.1016/0014-5793(83)81123-5. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Inagaki F., Miyazawa T. Advanced nuclear magnetic resonance lanthanide probe analyses of short-range conformational interrelations controlling ribonucleic acid structures. Biochemistry. 1981 May 12;20(10):2981–2988. doi: 10.1021/bi00513a041. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Yamaizumi Z., Nishimura S., Miyazawa T. 1H NMR studies on the conformational characteristics of 2-thiopyrimidine nucleotides found in transfer RNAs. Nucleic Acids Res. 1979 Jun 11;6(7):2611–2626. doi: 10.1093/nar/6.7.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]


