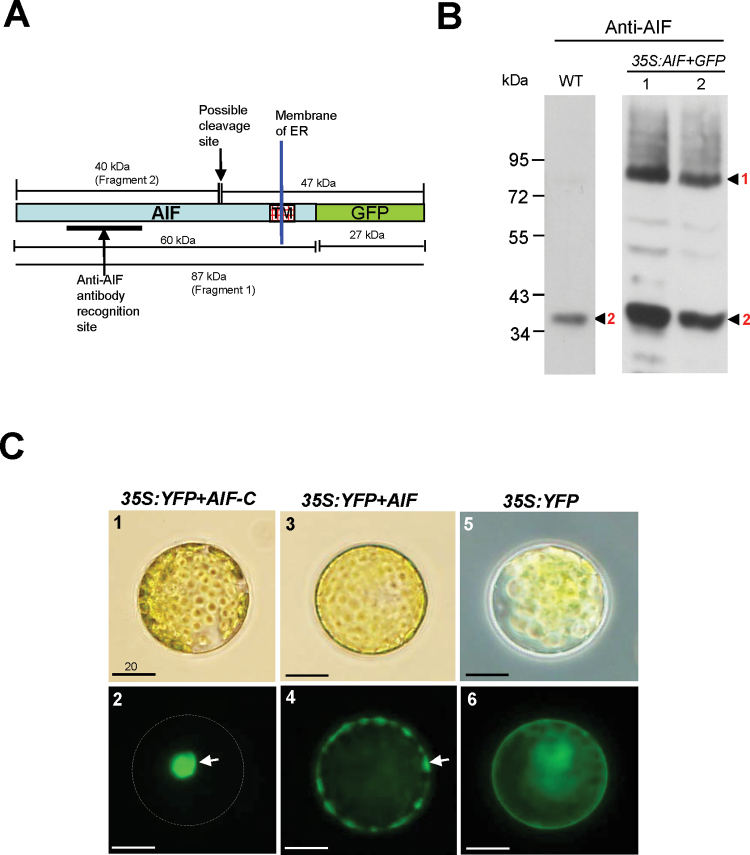
Fig. 3.
Detection of AIF proteins in flowers and the localization of AIF proteins in Arabidopsis protoplasts. (A) The protein structure of an uncleaved 87kDa AIF+GFP (Fragment 1) that contains the fusion of the proteins AIF (60kDa) and GFP (27kDa). Two peptides, 40kDa (Fragment 2) and 47kDa, will be produced after the AIF+GFP protein is processed, and the 40kDa Fragment 2 (AIF-C) will be released from the ER. The anti-AIF antibody recognition site and the possible cleavage site for AIF are indicated by arrows. The transmembrane domain (TM) in the C-terminus of the AIF protein is boxed. (B) The detection by western blot analysis of various forms of peptides for cleaved and uncleaved AIF+GFP in flowers. In wild-type flowers, only a cleaved 40kDa AIF-C band (Fragment 2) was detected by the anti-AIF antibody. In 35S:AIF+GFP flowers, a cleaved 40kDa AIF-C band (Fragment 2) and an uncleaved 87kDa AIF+GFP band (Fragment 1) were detected by the anti-AIF antibody. (C) Arabidopsis protoplasts transfected with 35S:YFP+AIF-C (C-1), 35S:YFP+AIF (C-3), and 35S:YFP (C-5). YFP+AIF-C (C-2), YFP+AIF (C-4), and YFP (C-6) fluorescence images of the transfected protoplasts from C-1, C-3, and C-5, respectively. The YFP+AIF-C fusion proteins accumulate in the nucleus (arrow) of the cell, and the corresponding cell membrane is indicated by the dashed circle (C-2). The YFP+AIF fusion proteins accumulate in the membrane (arrow) and are absent from the nucleus (C-4). The YFP proteins are dispersed in the cytoplasm (C-6). Bar=20 μm.