Abstract
The adeno-associated viral (AAV) vector has emerged as an attractive vector for gene therapy applications. Development of AAV vectors with enhanced gene transduction efficiency is important to ease the burden of AAV production and minimize potential immune responses. Rational mutations on AAV capsids have gained attention as a simple method of enhancing AAV transduction efficiency. A single-amino acid mutation, K137R, on AAV1 and AAV8 was recently reported to increase liver transgene expression by 5–10-fold. To determine whether the same mutation on other AAV serotypes would result in similar gene enhancement effects, K137R mutants were generated on AAV7, AAV8, and AAV9, and their effects were evaluated in vivo. Two reporter genes were utilized: the nuclear LacZ gene driven by the cytomegalovirus promoter and the luciferase gene driven by the CB promoter. Surprisingly, we found no difference in luciferase gene expression in the liver or other tissues using either the wild-type AAV8 capsid or AAV8-K137R. LacZ gene expression in the liver by AAV8-K137R was about onefold higher than that of wild-type AAV8. However, no difference was found in other tissues, such as skeletal muscle and cardiac muscle. In addition, no difference was found in transgene expression with either AAV7-K137R or AAV9-K137R mutants. Our results indicated that the K137R mutation on AAV7, AAV8, and AAV9 had minimal to no effect on transduction efficiency in vivo.
Introduction
Adeno-associated viral (AAV) vectors have been successfully applied in phase I clinical trials in patients with Leber congenital amaurosis and hemophilia B. The benefits of using AAV include the lack of pathogenicity, the broad tissue tropism, and long-term transgene expression (Daya and Berns, 2008). A number of clinical trials utilizing AAV have been carried out worldwide (Manno et al., 2003; Kaplitt et al., 2007; Maguire et al., 2008, 2009; Mueller and Flotte, 2008), and several challenges have been identified based upon their results (Grieger and Samulski, 2012). These challenges include tissue-specific tropism of AAV vectors, high quantity and high quality of recombinant AAV vectors, and immune response to AAV capsids and transgenes (Grieger and Samulski, 2012). To ease the burden of AAV production and minimize the immune response against AAV capsids, it is important to develop AAV vectors that provide enhanced gene expression at significantly low vector doses to achieve successful gene transfer in patients.
The AAV capsid influences transduction efficiency of the AAV vector at several steps, including vector binding to cell surface receptors, internalization, cytoplasmic trafficking to the nuclear membrane, vial uncoating, and second-strand synthesis. One of the most effective methods used to improve AAV transduction efficiency is using alternative AAV serotypes. Currently, at least 12 naturally occurring serotypes of AAV have been isolated (Gao et al., 2002; Wu et al., 2006; Grieger and Samulski, 2012). Different AAV serotypes exhibit distinct tissue tropism and interact with different cellular receptors (Zincarelli et al., 2008). In addition to the utilization of isolated naturally occurring AAV serotypes, generation of novel AAV variants with capsid libraries (Schaffer and Maheshri, 2004; Muzyczka and Warrington, 2005; Yang et al., 2009) and rational designs (Asokan et al., 2010; Bowles et al., 2012) are also important ways to expand AAV capsid versatility and improve AAV vector transduction efficiency.
One such strategy of rationally designed AAV was tyrosine-mutant AAVs (Zhong et al., 2008a). This strategy was based upon previous observation that epidermal growth factor receptor protein tyrosine kinase negatively affects AAV2 vector transduction by phosphorylation of tyrosine in the viral capsid, leading to the capsid being targeted for proteasomal degradation and ultimately preventing nuclear localization of the vector (Zhong et al., 2007). Zhong et al. (2008a) hypothesized that mutations of the surface-exposed tyrosine residues might allow the vectors to evade phosphorylation, subsequent ubiquitination, and prevent proteasome-mediated degradation. Seven surface-exposed residues were chosen for mutagenesis on the AAV2 vector. Y445F and Y731F showed the greatest impact on transduction efficiency with up to 29-fold gene expression increase observed in mouse hepatocytes after intravenous administration (Zhong et al., 2008a). However, the transduction enhancement mediated by tyrosine mutation appeared to be AAV serotype specific or dependent on tissue type and route of administration (Qiao et al., 2012). For example, the Y to F mutants of AAV8 and AAV9 have been reported to variably increase gene expression in the eye and brain (Petrs-Silva et al., 2009; Dalkara et al., 2011; Pang et al., 2011), but not in the skeletal or cardiac muscle (Qiao et al., 2012).
In an effort to search for simple and efficient method to improve transduction efficiency of AAV vector, Gabriel et al. (2013) hypothesized that the mutation of surface-exposed amino acids other than tyrosine on AAV capsid may facilitate AAV intracellular trafficking. Specifically, eight serine (S), seven threonine (T), and nine lysine (K) residues on AAV2 capsid were mutated to alanine (A) and arginine (R) individually or in combination. Targeted modifications of those amino acids were thought to circumvent the host-cellular serine/threonine kinase phosphorylation and ubiquitination of the viral capsids. Indeed, delivering the mutated AAV2 vector in vivo by tail vein injection resulted in higher vector copy numbers (up to 4.9-fold) and transgene expression in hepatocytes (up to 14-fold) (Gabriel et al., 2013). This strategy was also applied to the other serotypes by the same group such as AAV1, AAV5 (Sen et al., 2013a), and AAV8 (Sen et al., 2013b), and similar increases of gene transduction efficiency (up to 16-fold) were observed. In particular, the single–amino acid 137 K-R mutation on AAV1 (AAV1-K137R) and AAV8 (AAV8-K137R) resulted in consistently higher hepatocyte transgene expression in vivo with a 5–10-fold increase compared with the original capsids (Sen et al., 2013a,b). Therefore, the single–amino acid 137K-R mutation on AAV capsids was proven to be a simple and efficient method to improve AAV transduction efficiency in vivo. We set out to study whether the current mutant, such as AAV8-K137R, will mediate similar gene expression enhancement effects in organs other than the liver. In addition, we also wanted to examine whether a mutation on other serotypes such as AAV9 will have comparable gene transfer enhancement effects, as AAV9 is a better option for muscle-directed gene therapy (Inagaki et al., 2006; Kornegay et al., 2010).
Materials and Methods
Generation of K137R AAV plasmids and AAV vector production
The original AAV9 plasmid was obtained from Dr. J. Wilson laboratory of the University of Pennsylvania (Philadelphia, PA) (Bish et al., 2008). The AAV7 and AAV8 plasmids were commercially synthesized (Wang et al., 2005). The K-R mutant packaging plasmids were generated via site-specific mutagenesis. The primers were as follows: AAV7-K137R(+): ACG GCT CCT GCA AAG AAG AGA C; AAV8-K137R(+): ACG GCT CCT GGA AAG AAG AGA C; AAV7 and AAV8-K137R(−): CCT AGC GCC TTC CTC AAC CAG AC; AAV9-K137R(+): ACG GCT CCT GGA AAG AAG AGG C; AAV9-K137R(−): CCT AGC CGC TTC CTC AAC CAG AC. Recombinant AAV vectors were generated by the triple-plasmid transfection method (Xiao et al., 1998). Viral vectors were purified by polyethylene glycol precipitation followed by double CsCl gradient purification (Ayuso et al., 2010). After three time changes of dialysis in virus dialysis buffer (1× phosphate-buffered saline, 25 mannitol, 6 mM MgCl2) at room temperature for 6–8 hr or at 4°C for overnight, vector genome titers were determined by DNA dot blot and confirmed by quantitative polymerase chain reaction (PCR).
In vivo gene transfer
All animal experiments were approved by the Institutional Animal Care and Use Committee. The breeding pairs of ICR mice were purchased from Taconic (Hudson, NY), and C57BL6 and BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME). Four or five mice were included in each group.
Vector genome copy number determination
Total DNA was extracted with a kit (DNeasy blood and tissue kit, cat. no. 69506; Qiagen, Valencia, CA). Vector copy number was determined with a 7300 real-time PCR system (Applied Biosystems, Foster City, CA). TaqMan assays for both endogenous control (glucagon gene) and AAV vector were developed as described elsewhere (Qiao et al., 2012).
Luciferase imaging and luciferase activity assay
BALB/c mice at the age of 6 weeks received 1×1011 particles of AAV8/luc via retro-orbital injection. One week later, images were taken using a Xenogen IVIS Lumina imaging system (Caliper Life Sciences–PerkinElmer, Hopkinton, MA) after intraperitoneal injection of d-luciferin substrate (120 mg/kg; NanoLight Technology, Pinetop, AZ). Bioluminescence image was analyzed with Living Image software (PerkinElmer, Waltham, MA).
At week 2 after AAV/luc injection, mice were euthanized and different tissues were harvested for in vitro luciferase activity assay. Luciferase activity in tissues was determined using a luminometric assay (Promega, Madison, WI) and normalized to the total recovered protein determined by the Bradford assay.
Statistical analysis
Data were presented as means±standard deviation. Comparison between two groups was analyzed with the Student's t-test; p<0.05 was considered statistically significant.
Results
K137R mutants of AAV7, AAV8, and AAV9 in ICR mice with LacZ reporter
To investigate whether the simple amino acid mutation method would reproduce the impressive gene expression enhancement for other serotypes, we created AAV7-K137R and AAV9-K137R, in addition to the AAV8-K137R packaging plasmid (see Materials and Methods for details) (Sen et al., 2013b). The mutations were independently confirmed by DNA sequencing. The original and K137R mutant of AAV7, AAV8, and AAV9 vectors were produced containing the nuclear LacZ (LacZnls) gene driven by a ubiquitous cytomegalovirus (CMV) promoter. The LacZ gene was chosen as a reporter because it could be easily visualized in transduced tissue and conveniently quantified by β-galactosidase activity in the tissue (Qiao et al., 2012). No difference was found in AAV yields among all six vectors generated, indicating that the point mutations did not compromise the vector packaging capacity (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hgtb).
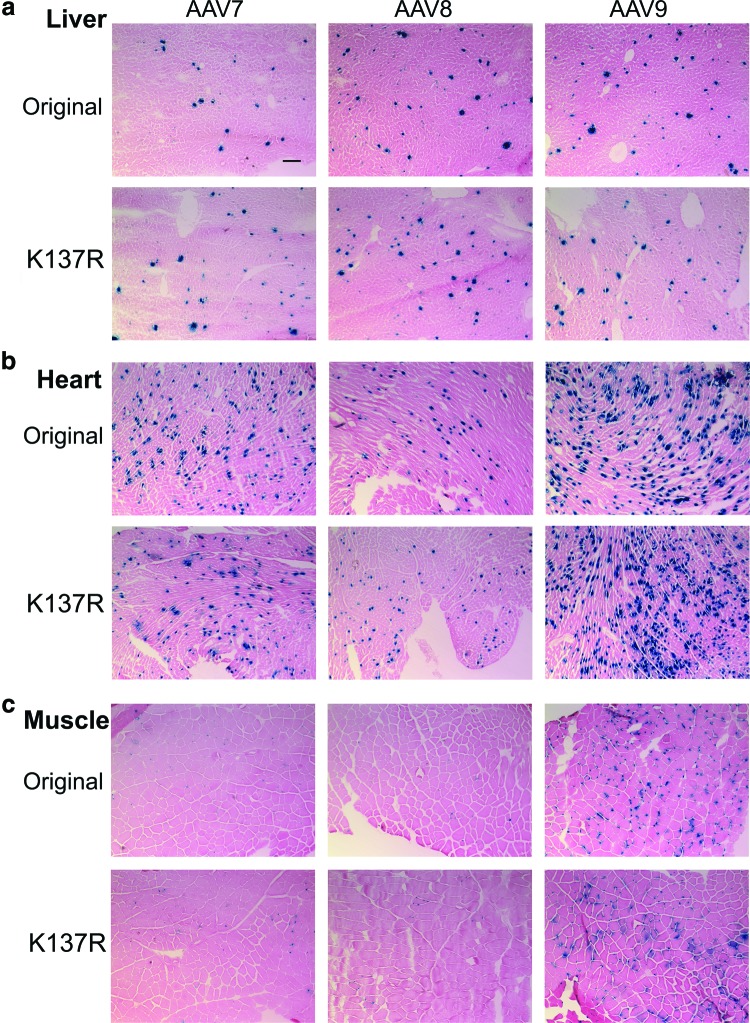
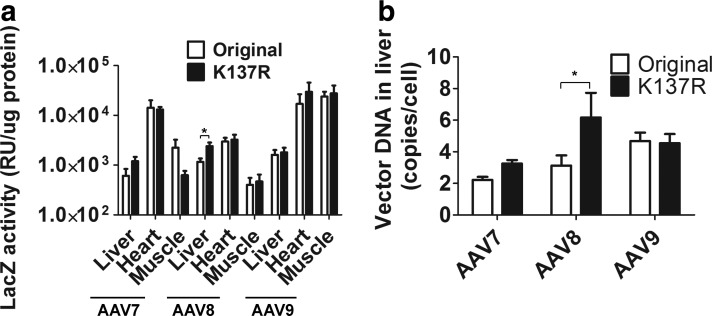
The AAV vectors (5×1011 vg/mouse) were delivered into 4-week-old ICR mice via tail vein injection (n=5 for each group), and all mice were euthanized within 2 weeks postinjection. We chose to deliver AAV vectors in young adult mice, as this is an efficient way to achieve skeletal muscle and cardiac muscle gene transfer in addition to hepatic gene expression. First, different tissues were cryo-sectioned and subjected to LacZ staining. As shown in Fig. 1, only a moderate increase of LacZ expression in liver was observed by LacZ staining for the AAV8-K137R group as compared with the original AAV8 group (Fig. 1a). There was no apparent difference of LacZ expression in the liver for AAV9-K137R versus AAV9 group and AAV7-K137R versus AAV7 group (Fig. 1a). Aside from the liver, we focused on the muscle (TA muscle) and heart to see whether the K-R mutation could enhance gene expression in these tissues. As shown in Fig. 1, no significant difference in LacZ expression was found in either the heart or the muscle for any of the compared serotypes (AAV7, AAV8, and AAV9) (Fig. 1b and c). Next, LacZ activity from different tissues was measured and quantified. The quantitation data were relatively consistent with the LacZ staining data. A moderate difference (an increase by 1-fold) of LacZ activity was observed only in the liver for the AAV8-K137R group (2,404±995 RU/μg protein) as compared with the original AAV8 (1,156±454 RU/μg protein) group (Fig. 2a; n=5, p<0.05). The increase of LacZ activity in the liver was not apparent for AAV7-K137R versus AAV7 and for AAV9-K137R versus AAV9 (Fig. 2a). Similar to the LacZ staining results, there was no significant difference of LacZ activity in the cardiac muscle (Fig. 2a) or the skeletal muscle (Fig. 2a; n=5, p>0.05). Finally, total DNA was extracted from the liver, and quantitative PCR was performed to determine AAV vector DNA amounts. The higher AAV vector DNA copy numbers were expected in the mutant groups, since the K137R mutation on AAV capsid was supposed to decrease capsid ubiquitination and improve intracellular trafficking of AAV DNA (Sen et al., 2013b). Indeed, the vector DNA of hepatocytes was doubled in the AAV8-K137R group (6.17±2.7 copies/cell) as compared with the AAV8 group (3.12±1.11 copies/cell) (Fig. 2b). However, there was no significant difference of vector DNA copy number of hepatocytes for AAV7-K137R (3.25±0.38 copies/cell) versus AAV7 (2.22±0.32 copies/cell) and for AAV9-K137R (4.54±1.0 copies/cell) versus AAV9 (4.67±0.92 copies/cell) (Fig. 2b).
FIG. 1.
Comparison of original and K137R mutants of AAV7-, AAV8-, and AAV9-cmv-LacZnls vectors in ICR mice via LacZ staining. About 5×1011 vg of AAV vectors were delivered into 1-month-old ICR male mice via tail vein injection, and the mice were euthanized 2 weeks postinjection (n=5 for each group). (a) LacZ staining of liver tissue. The nucleus LacZ was stained blue (scale bar: 100 μm). (b) LacZ staining of heart. (c) LacZ staining of muscle. AAV, adeno-associated virus. Color images available online at www.liebertpub.com/hgtb
FIG. 2.
Quantitation of LacZ activity and vector DNA copy numbers. (a) Quantitation of LacZ activity (*p<0.05, and the Student's t-test was applied for the statistics). (b) AAV vector DNA copy numbers in liver tissue.
K137R mutants of AAV7, AAV8, and AAV9 in BL6 mice with LacZ reporter
Different genetic background of mice may occasionally lead to different outcomes of AAV gene transfer. Therefore, we evaluated the K-R mutated vectors in inbred C57BL/6 mice, in addition to ICR mice. K-R mutant and corresponding original AAV vectors (2×1011 vg/mouse) were injected into 3-month-old C57BL/6 mice via tail vein injection. This time the less vector particles were utilized to rule out gene expression saturation issue, and the treated mice were euthanized earlier to avoid the concern of CMV promoter shutting off in the liver because of longer gene expression time (Qiao et al., 2011). The treated mice were euthanized 1 week postinjection, and various tissues were cryo-preserved. We analyzed only the liver tissue because very limited gene expression appeared in the muscle and heart with a low dose of the AAV vector. Overall, the data obtained from the C57BL/6 mice were in agreement with the ICR mice data. A slight increase of LacZ expression in the liver was observed in the AAV8-K137R group as compared with the original AAV8 group (Supplementary Fig. S2a). Also, LacZ activity displayed slight increases (by 1.7-fold differences) in expression in the liver for the AAV8-K137R group (742±144 RU/μg protein) compared with the original AAV8 group (270±219 RU/μg protein) (Supplementary Fig. S2a; n=4–5, p<0.05). The LacZ expression did not show dramatic increases in hepatocytes for either AAV7-K137R group or AAV9-K137R group (Supplementary Fig. S2a and b; n=5, p>0.05).
AAV8-K137R mutant with luciferase reporter
A previous study indicated that the K137R-mutated AAV8 vector resulted in a 10-fold increase of hepatic gene transfer compared with the wild-type AAV8 (Sen et al., 2013b); however, our current data showed only moderate increase of hepatic gene transfer. These conflicted results prompted us to repeat the similar experiments in a different lab using a different reporter gene. Therefore, the AAV8-K137R packaging plasmid was re-created and used to package luciferase transgene, which was driven by the chicken β-actin promoter with CMV enhancer (CB), because the CB promoter has been recognized to render long-term efficient gene expression in vivo in many tissues, including the liver (Xu et al., 2001; Wang et al., 2003). The AAV8 and AAV8-K137R vectors (1×1011 particles) were delivered into the 6-week-old Balb/C mice via retro-orbital injection (n=4–5 per group). The live mice were subjected to optimal image study to examine luciferase intensity at week 1 postinjection. As shown in Fig. 3a, the optimal image did not display significant differences in luciferase intensity between the AAV8 and AAV8-K137R group. The mice were euthanized at week 2 postinjection, and various tissues, including heart, spleen, liver, lung, kidney, and muscle (gastrocnemius), were carefully dissected and subjected to luciferase activity assay. Again, we failed to see any significant difference in luciferase expression between mutated AAV8-K137R and original AAV8 groups for all the tissues examined (Fig. 3b; n=4–5, p>0.05).
FIG. 3.
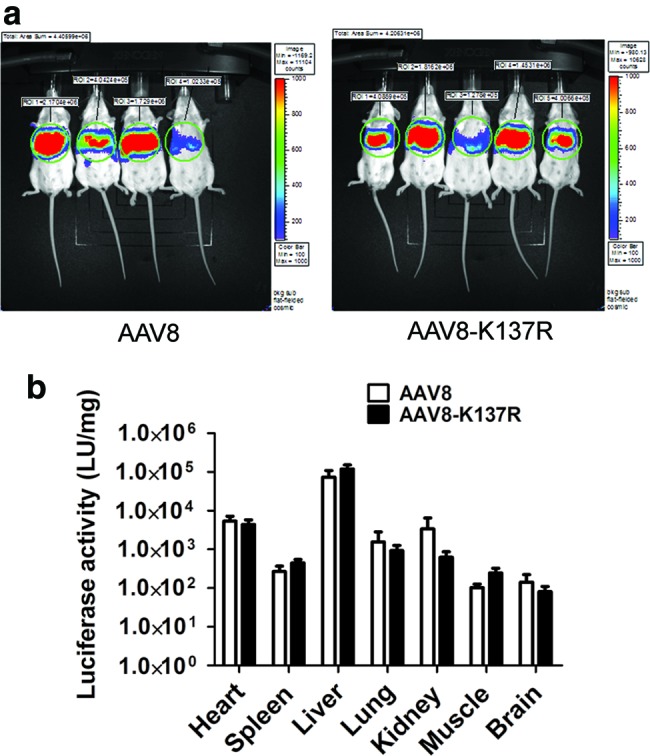
Transgene luciferase expression after systemic administration of AAV/luc vector. About 1×1011 particles of the AAV8/luc or AAV8-K137R vector were administered into 6-week-old Balb/C mice via retro-orbital injection. At week 1 after AAV vector injection, the imaging was performed with 10 sec exposure after injection of d-luciferin substrate (a) At week 2, different tissues were collected and luciferase activity was measured; relative light units of luciferase levels are shown as normalized to protein levels determined using a Bradford assay. Error bars indicates standard deviation (b). Color images available online at www.liebertpub.com/hgtb
Discussion
AAV vector has been recognized as an attractive vector for gene therapy applications (Manno et al., 2003; Kaplitt et al., 2007; Hauswirth et al., 2008; Maguire et al., 2009; Nathwani et al., 2011). Our lab has a long-standing interest in developing AAV vectors for muscle-directed gene therapy application, such as gene therapy for muscular dystrophies (Wang et al., 2000; Qiao et al., 2005; Zhu et al., 2005). Muscular dystrophy gene therapy requires therapeutic gene transfer to most of the striated muscle cells in a body-wide manner to achieve desirable efficacies in patients. Because high vector doses are often required in large animals (Kornegay et al., 2010) and eventually in human patients (Mendell et al., 2010), improvement in every step of gene transfer process counts. The tyrosine-to-phenylalanine mutation was intended to overcome one of the rate-limiting steps in AAV infection, that is, intracellular trafficking. Mutation of the tyrosine residues on the capsid might allow the vectors to evade phosphorylation mediated by tyrosine kinase and subsequent ubiquitination. Therefore, more vector can emerge out of the trafficking pathway and enter the nucleus for transgene expression (Zhong et al., 2008b). Mutation of serine/threonine to alanine or lysine (K) to arginine (R) on AAV capsid shares the same principle as tyrosine mutation. The rationale is to alter the enzymatic (kinase/ubiquitination ligase) targets on AAV capsid to circumvent capsid ubiquitination and increase the transduction efficiency of these vectors (Gabriel et al., 2013). This strategy was originally attempted on AAV2 capsid, and the mutant AAV2 vector resulted in the increase of transgene expression up to 14-fold (Gabriel et al., 2013). Later, AAV1, AAV5 (Sen et al., 2013a), and AAV8 (Sen et al., 2013b) were mutated, making it appear that most of the mutant vectors mediated much higher transgene expression than the original capsid. In particular, the AAV8-K137R mutant resulted in a significantly higher vector copy numbers in the liver (22-fold), lungs (9.7-fold), and muscle (8.4-fold) tissues when compared with the original AAV8 vectors (Sen et al., 2013b). AAV1-K137R also demonstrated much higher (up to sixfold) luciferase expression. Since the lysine K137 residue was conserved across most of the AAV serotypes, we hypothesized that K137R mutation on other serotypes would result in the similar gene enhancement effects.
K137R mutants were generated on AAV7, AAV8, and AAV9 vectors. Two reporter genes were utilized: the nuclear LacZ gene driven by CMV promoter and luciferase gene driven by CB promoter. Several mouse strains, including Balb/C, ICR, and C57BL/6, were included. The experiments were even performed in two independent labs. Quite surprisingly, we did not observe the dramatic gene enhancement effects for any of the K137R mutants. Only moderate increase of transgene expression (up to 1.7-fold) in the liver was noticed for AAV8-K137R containing LacZ reporter. We did not observe any significant gene enhancement effects on other tissues such as skeletal muscle and cardiac muscle for AAV8-K137R. No significant increase of transgene expression was found in any of the tissues for AAV7-K137R and AAV9-K137R. The results were somewhat disappointing, but nonetheless informative and useful.
Some possible explanations for the conflicting results of our current study (an increase by 1-fold) and those of others (10-fold increase) (Sen et al., 2013b) were deduced. The same mutant AAV8-K137R was used in both studies. However, they had a different reporter. In our study, both nuclear LacZ and luciferase reporters were used, whereas GFP reporter was used in the other report (Sen et al., 2013b). The sensitivity of different reporters might contribute to various fold changes of transduction efficiency. Other factors, including incorrect vector titers, dilutions, and inconsistent vector potency from batch to batch, could also cause inconsistent results.
Nevertheless, tyrosine/serine/threonine mutation on AAV capsids represents a novel and distinct strategy to enhance AAV transduction efficiency by improving intracellular trafficking of AAV capsids. However, caution needs to be taken when applying this strategy, since it is both serotype specific and tissue specific. If a certain serotype of AAV in a given tissue/cell type is already efficient in this process, one would not expect significant improvement after all. For example, both AAV8 and AAV9 are the most robust vectors for liver gene delivery. Also, AAV8 has been found to be effective in intracellular trafficking and uncoating (Nakai et al., 2005). On the other hand, AAV2 is ineffective in the above-mentioned processes in the liver and therefore poor in hepatic gene transfer, despite the fact that its receptor binding and entry into liver cells are nearly as effective as those of AAV8 (Thomas et al., 2004). Given our observation that the K137R mutation on AAV7 and AAV9 did not increase transgene expression, we speculate that intracellular trafficking, in particular the escape of proteasomal degradation, may not be the rate-limiting step for these two vectors in the studied tissues.
Supplementary Material
Acknowledgments
We thank Carrie Martin (PharmD candidate, UNC Eshelman School of Pharmacy) for her critical reading of this article. This study was supported by the following National Institutes of Health grants: 1RO1 NS082536, 1RO1NS079568, and 5RO1AR056394 (X.X.); 1R01AI080726 and 5R01DK084033 (C.L. and R.J.S.); and 5U54AR056953 (R.J.S).
Author Disclosure Statement
No competing financial interests exist.
References
- Asokan A., Conway J.C., Phillips J.L., et al. (2010). Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 28, 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso E., Mingozzi F., Montane J., et al. (2010). High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 17, 503–510 [DOI] [PubMed] [Google Scholar]
- Bish L.T., Morine K., Sleeper M.M., et al. (2008). Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum. Gene Ther. 19, 1359–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D.E., Mcphee S.W., Li C., et al. (2012). Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 20, 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D., Byrne L.C., Lee T., et al. (2011). Enhanced gene delivery to the neonatal retina through systemic administration of tyrosine-mutated AAV9. Gene Ther. 19, 176–181 [DOI] [PubMed] [Google Scholar]
- Daya S., and Berns K.I. (2008). Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 21, 583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel N., Hareendran S., Sen D., et al. (2013). Bioengineering of AAV2 capsid at specific serine, threonine, or lysine residues improves its transduction efficiency in vitro and in vivo. Hum. Gene Ther. Methods 24, 80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G.P., Alvira M.R., Wang L., et al. (2002). Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99, 11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger J.C., and Samulski R.J. (2012). Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. 507, 229–254 [DOI] [PubMed] [Google Scholar]
- Hauswirth W.W., Aleman T.S., Kaushal S., et al. (2008). Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 19, 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K., Fuess S., Storm T.A., et al. (2006). Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 14, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt M.G., Feigin A., Tang C., et al. (2007). Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 369, 2097–2105 [DOI] [PubMed] [Google Scholar]
- Kornegay J.N., Li J., Bogan J.R., et al. (2010). Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol. Ther. 18, 1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., High K.A., Auricchio A., et al. (2009). Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 374, 1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., Simonelli F., Pierce E.A., et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S., Chew A.J., Hutchison S., et al. (2003). AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101, 2963–2972 [DOI] [PubMed] [Google Scholar]
- Mendell J.R., Campbell K., Rodino-Klapac L., et al. (2010). Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 363, 1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., and Flotte T.R. (2008). Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 15, 858–863 [DOI] [PubMed] [Google Scholar]
- Muzyczka N., and Warrington K.H., Jr., (2005). Custom adeno-associated virus capsids: the next generation of recombinant vectors with novel tropism. Hum. Gene Ther. 16, 408–416 [DOI] [PubMed] [Google Scholar]
- Nakai H., Fuess S., Storm T.A., et al. (2005). Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J. Virol. 79, 214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani A.C., Tuddenham E.G., Rangarajan S., et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J.J., Dai X., Boye S.E., et al. (2011). Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol. Ther. 19, 234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrs-Silva H., Dinculescu A., Li Q., et al. (2009). High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 17, 463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C., Li J., Zhu T., et al. (2005). Amelioration of laminin-alpha2-deficient congenital muscular dystrophy by somatic gene transfer of miniagrin. Proc. Natl. Acad. Sci. USA 102, 11999–12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C., Yuan Z., Li J., et al. (2011). Liver-specific microRNA-122 target sequences incorporated in AAV vectors efficiently inhibits transgene expression in the liver. Gene Ther. 4, 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C., Yuan Z., Li J., et al. (2012). Single tyrosine mutation in AAV8 and AAV9 capsids is insufficient to enhance gene delivery to skeletal muscle and heart. Hum. Gene Ther. Methods 23, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer D.V., and Maheshri N. (2004). Directed evolution of AAV mutants for enhanced gene delivery. Conf. Proc. IEEE Eng. Med. Biol. Soc. 5, 3520–3523 [DOI] [PubMed] [Google Scholar]
- Sen D., Balakrishnan B., Gabriel N., et al. (2013a). Improved adeno-associated virus (AAV) serotype 1 and 5 vectors for gene therapy. Sci. Rep. 3, 1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Gadkari R.A., Sudha G., et al. (2013b). Targeted modifications in adeno-associated virus serotype 8 capsid improves its hepatic gene transfer efficiency in vivo. Hum. Gene Ther. Methods 24, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.E., Storm T.A., Huang Z., and Kay M.A. (2004). Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 78, 3110–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li J., and Xiao X. (2000). Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. USA 97, 13714–13719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Ma H.I., Li J., et al. (2003). Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 10, 2105–2111 [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhu T., Qiao C., et al. (2005). Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 23, 321–328 [DOI] [PubMed] [Google Scholar]
- Wu Z., Asokan A., and Samulski R.J. (2006). Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14, 316–327 [DOI] [PubMed] [Google Scholar]
- Xiao X., Li J., and Samulski R.J. (1998). Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72, 2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Daly T., Gao C., et al. (2001). CMV-beta-actin promoter directs higher expression from an adeno-associated viral vector in the liver than the cytomegalovirus or elongation factor 1 alpha promoter and results in therapeutic levels of human factor X in mice. Hum. Gene Ther. 12, 563–573 [DOI] [PubMed] [Google Scholar]
- Yang L., Jiang J., Drouin L.M., et al. (2009). A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc. Natl. Acad. Sci. USA 106, 3946–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Li B., Jayandharan G., et al. (2008a). Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology 381, 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Li B., Mah C.S., et al. (2008b). Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. USA 105, 7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L., Zhao W., Wu J., et al. (2007). A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol. Ther. 15, 1323–1330 [DOI] [PubMed] [Google Scholar]
- Zhu T., Zhou L., Mori S., et al. (2005). Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation 112, 2650–2659 [DOI] [PubMed] [Google Scholar]
- Zincarelli C., Soltys S., Rengo G., and Rabinowitz J.E. (2008). Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 16, 1073–1080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.