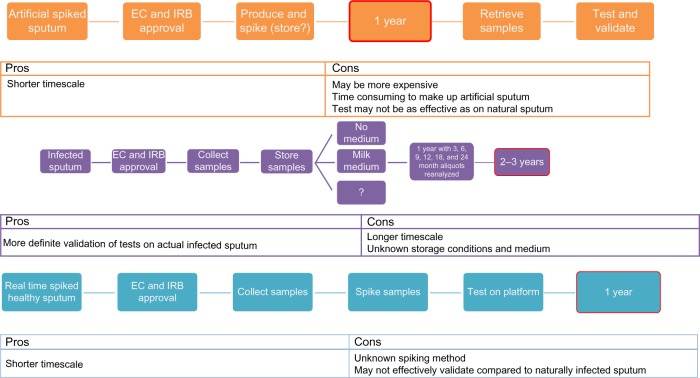
Figure 2.
Timelines to proof of concept of rapid diagnostic test.
Notes: Depending on the sample method chosen, timelines can be vastly increased or decreased. Spiked or real time samples could mean that devices could be validated within 1 year, while attempting to validate artificial sputum samples first could delay the project by at least 1 year. Similarly, attempting a validation study on the long-term storage of infected sputum samples, which again may or may not be successful, could cause validation of a rapid POCT to be delayed by anywhere up to 3 years.
Abbreviations: EC, ethics committee; IRB, institutional review board; POCT, point of care test.