Abstract
This paper describes a novel formulation of antineoplastic drug: mitoxantrone loaded into liposomal carriers enriched with encapsulated anacardic acid in the liposomal bilayer using a vitamin C gradient. Anacardic acid is a potent epigenetic agent with anticancer activity. This is the first liposomal formulation to combine an actively encapsulated drug and anacardic acid. The liposomes were characterized in terms of basic parameters, such as size, zeta potential, optimal drug-to-lipid ratio, loading time and temperature, and stability at 4°C and in human plasma in vitro. The formulation was found to be stable, and the loading process was rapid and efficient (drug-to-lipid ratio of up to 0.3 with over 90% efficiency in 5 minutes). The cytotoxicity of these formulations was assessed using the human melanoma cell lines A375 and Hs294T and the normal human dermal fibroblast line. The results showed that anacardic acid and to a smaller extent vitamin C significantly increased the cytotoxicity of the drug towards melanoma compared to ammonium sulfate liposomes. On the other hand, vitamin C and anacardic acid both protected normal cells from damage caused by the drug. The formulation combining anacardic acid, vitamin C, and mitoxantrone showed promising results in terms of cytotoxicity and cytoprotection. Therefore, it has potential for anticancer treatment.
Keywords: anacardic acid, vitamin C, ascorbic acid, liposomes, mitoxantrone, melanoma
Introduction
2-Hydroxy-6-pentadecylbenzoic acid (Figure 1) is one of the homologues of anacardic acid (AA). It is present in cashew nutshell liquid (CNSL) as a mixture of homologues with constant side-chain length (C15), but with a variable degree of unsaturation. AA has become a compound of interest in recent years due to its anticancer properties. It was shown to inhibit p300, which has histone acetyltransferase activity.1 This enzyme is involved in the regulation of the transcription of genes responsible for deoxyribonucleic acid (DNA) damage and repair, so it is a target for epigenetic cancer treatment.2 Inhibition of p300 was shown to inhibit the nuclear factor (NF)-κB pathway that regulates cell proliferation, survival, and inflammation.3 It was also demonstrated that anacardic acid suppressed vascular endothelial growth factor-driven angiogenesis, resulting in tumor-growth inhibition.4 Its activity has been shown on melanoma5 and colon, breast, lung, cervical, and kidney cancers.6 It can also inhibit the growth of hormone-dependent tumors, by suppressing the activity of androgen7 and estrogen8 receptors. Due to the amphipathic properties of AA, it can be incorporated easily into the liposome bilayer for the purpose of delivery.
Figure 1.
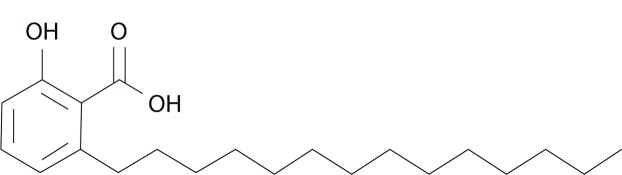
Structure of anacardic acid (C15:0).
In this study, we loaded preformed AA-containing liposomes with mitoxantrone (MIT). MIT is a synthetic anthracenedione derivative designed in the 1970s as an antitumor drug with lower toxicity than regular anthracyclines (eg, doxorubicin), especially in terms of cardiotoxicity.9 The cardiotoxic activity of anthracyclines is mainly related to the generation of reactive oxygen species (ROS). MIT was found to generate less ROS than doxorubicin, and even terminate free radical lipid peroxidation.10 Nevertheless, a fraction of patients suffer from late cardiotoxicity after MIT treatment, most probably due to MIT metabolites.11 Other side effects include bone marrow suppression, leukopenia, alopecia, infertility, and leukemia.12 The exact mechanism of MIT action involves inhibiting DNA and ribonucleic acid synthesis by intercalation and electrostatic interactions with nucleic acids.13,14 These interactions lead to single- or double-strand breaks. MIT was also shown as an inhibitor of topoisomerase II, an enzyme crucial for DNA replication and repair processes.15 The cytotoxicity of MIT is independent of the phase of the cell cycle, so it can be effective against slow-growing tumors.16 Free MIT is used in the treatment of various cancers, such as breast cancer, acute nonlymphocytic leukemia, non-Hodgkin’s lymphoma,17 castration-resistant prostate cancer,18 and melanoma.19
We loaded MIT into liposomes modified with AA via a vitamin C gradient. Vitamin C was found to induce a specific toxicity against cancer cells20 while having a protective effect on normal cells.21 Additionally, vitamin C improved the anticancer properties of some anthracyclines, most notably doxorubicin,22 probably in a synergistic manner. Our previous research demonstrated that epirubicin loaded via a vitamin C gradient also has promising anticancer properties against breast cancer, both in vitro and in vivo,23 superior to those of epirubicin loaded via an ammonium sulfate (AS) gradient.
Long-circulating liposomes are one of the most widely applied intravenous drug delivery systems. Encapsulated doxorubicin (Doxil®/Caelyx®) yielded better pharmacokinetic parameters and superior anticancer activity,24 so we expect to enhance the efficacy of MIT in a similar way. However, only a few liposomal formulations of MIT have been proposed so far,25–27 and their clinical application is limited.
In our research, we intended preliminarily to study the influence of AA on the basic parameters of liposomes loaded with MIT via a vitamin C gradient, such as the size and zeta potential of vesicles, optimal drug-to-lipid ratio, loading time, and temperature. We also examined the storage stability of the formulation and drug release in human plasma. Most importantly, we assessed the effects of AA and vitamin C combined with MIT on the proliferation of melanoma and normal cells. To the best of our knowledge, this is the first reported case of an anticancer drug encapsulated in liposomes containing AA.
Materials and methods
Materials
Hydrogenated soy phosphatidylcholine (HSPC), 1,2-distearoyl-sn-glycero-phosphoethanolamine-N-(poly[ethylene glycol]2000) (DSPE-PEG2000) and cholesterol (CHOL) were purchased from Northern Lipids (Vancouver, BC, Canada). CNSL was obtained from Sandor Cashew (Delhi, India). Sodium dihydrogen phosphate, disodium hydrogen phosphate, sodium chloride, ascorbic acid, and ammonium hydroxide were purchased from Avantor Performance Materials (Gliwice, Poland). Sephadex G-50 fine and Sepharose CL-4B were obtained from Sigma-Aldrich (St Louis, MO, USA). MIT hydrochloride was a gift from the Pharmaceutical Research Institute (Warsaw, Poland). All the other reagents were of analytical grade.
Anacardic acid extraction and hydrogenation
AA was extracted using a method based on AA forming an insoluble salt with lead (II) hydroxide. Briefly, 10 g natural CNSL was dissolved in 80 mL methanol and stirred at 56°C for 1 hour with 3.8 g freshly prepared lead (II) hydroxide. At this point, the AA salt formed a precipitate, and the other components of CNSL remained in solution. The precipitate was washed with ethanol and dissolved in 1.3 M HCl.
AA was then extracted with ethyl acetate, dried, and dissolved in methanol to allow the hydrogenation of unsaturated bonds in the aliphatic side chain of AA. The dissolution used 50 mL methanol, then 10 mL hydrazine and 15 mL hydrogen peroxide were added. The hydrogenation was conducted at 50°C overnight with continuous stirring. After the reaction, the pH of the solution was adjusted to 5.5 with HCl, and AA was extracted with chloroform, dried under low pressure, and freeze-dried from cyclohexane. The purity of the hydrogenated AA was confirmed via high-performance liquid chromatography with the following parameters: mobile phase – methanol:water (85:15); flow rate − 1.75 mL/minute; and injection volume − 25 μL. The structure of isolated AA was confirmed by mass spectrometry and nuclear magnetic resonance analyses.
Preparation of liposomes
Liposomes were prepared by lipid hydration, a freeze-and-thaw procedure, and extrusion through polycarbonate filters.28 Chloroform stock solutions of lipids (HSPC, CHOL, DSPE-PEG2000, and AA) were prepared. Appropriate volumes were then transferred to borosilicate glass tubes to obtain the molar ratios of lipids shown in Table 1. Chloroform was evaporated under gas nitrogen, and the resulting thin lipid film was dissolved in cyclohexane with 0.1% (v/v) methanol. The mixture was subsequently frozen in liquid nitrogen and freeze-dried overnight at low pressure using a Savant Modulyo apparatus (Thermo Fisher Scientific, Waltham, MA, USA).
Table 1.
Liposome composition: molar ratios are given
| Liposomes | HSPC | CHOL | DSPE-PEG2000 | AA |
|---|---|---|---|---|
| CHOL liposomes | 0.55 | 0.40 | 0.05 | – |
| AA liposomes | 0.55 | 0.35 | 0.05 | 0.05 |
Abbreviations: HSPC, hydrogenated soy phosphatidylcholine; CHOL, cholesterol; DSPE-PEG, 1,2-distearoyl-sn-glycero-phosphoethanolamine-N-(poly[ethylene glycol]2000); AA, anacardic acid.
The lipids were then hydrated with 300 mM ammonium ascorbate (pH 4.0) or 300 mM ammonium sulfate, as indicated. The hydration process was conducted in a water bath at 65°C. The resulting multilamellar vesicles were subjected to ten cycles of freezing in liquid nitrogen and thawing in a water bath at 65°C.
After the freeze-and-thaw procedure, the liposomes were extruded ten times through Nucleopore polycarbonate filters with pore size of 100 nm on a Thermobarrel Extruder (PPHU Marker, Wrocław, Poland). The extruder was thermostated to 64°C prior to liposome extrusion. As a result, large unilamellar vesicles were formed. Their size, size distribution, and zeta potential were characterized afterwards.
Preparation of the ion and/or pH gradient for drug encapsulation
The ion and/or pH gradient was subsequently generated by exchanging the extravesicular liposomal solution on Sephadex G-50 (1 × 20 cm) columns equilibrated with phosphate-buffered saline consisting of 20 mM sodium phosphate and 150 mM NaCl (pH 7.4). The lipid concentration was determined in the collected liposomal fractions. MIT hydrochloride solution in 150 mM NaCl (6 mg/mL) was added to the large unilamellar vesicle suspension at drug-to-lipid molar ratios of 0.1, 0.2, 0.25, 0.3, and 0.5 to determine the encapsulation efficiency (EE) at different ratios. In all the other experiments, a drug-to-lipid ratio of 0.2 was used. In order to establish the optimal loading temperature, liposomes were incubated with MIT for 5 minutes at 52°C, 54°C, 56°C, 58°C, and 60°C. To determine the kinetics of drug encapsulation, 50 μL liposomal samples were taken after loading for 2.5, 5, 15, or 60 minutes. If not indicated otherwise, the loading process was conducted at a drug-to-lipid ratio of 0.2 for 5 minutes at 60°C.
Liposome characterization
Liposome size was determined via dynamic light scattering.29 Liposomes were diluted to a total lipid concentration of approximately 10 μM in 150 mM NaCl prior to size and zeta-potential measurements. The mean diameter (volume weighting), polydispersity index (PDI), and zeta potential of the prepared liposomes was measured using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). The phospholipid concentration was measured using the ammonium ferrothiocyanate assay30 on a Shimadzu UV 2401 PC spectrophotometer (Shimadzu, Kyoto, Japan). MIT concentration was measured at λ=667 nm on the same spectrophotometer after liposome dissolution in methanol.
Determination of encapsulation efficiency
After remote loading, nonencapsulated drug was separated from MIT-containing liposomes on Sephadex G-50 Fine minicolumns (5.5 × 70 mm) pre-equilibrated with 150 mM NaCl solution. Liposome samples (50 μL) were placed on a column, and free MIT was separated from MIT-containing liposomes. Then, the liposome fraction was collected and the concentrations of lipid and MIT were determined to enable calculation of EE according to the formula:
where initial D/L means the drug-to-lipid ratio at the point when the drug was mixed with the liposomes.
Long-term stability of liposomal MIT – preliminary study
In order to obtain preliminary information about the shelf life of the AA liposomes loaded with MIT via an ammonium ascorbate gradient at a drug-to-lipid ratio of 0.2, liposomes were stored at 4°C, and at selected times, 100 μL samples were placed on Sephadex G-50 Fine minicolumns (5.5 × 70 mm) pre-equilibrated with 150 mM NaCl solution to separate the liposomal fraction from the released drug. The MIT and lipid concentrations were measured in the collected liposomal fractions, and the resulting drug-to-lipid ratio was compared with the initial value. The liposomes for the experiment were diluted to a lipid concentration of 2 mM with 150 mM NaCl containing 0.1% NaN3.
MIT release from liposomes in the presence of human plasma in vitro
CHOL and AA liposomes containing MIT (drug-to-lipid ratio of 0.2) loaded via ammonium ascorbate gradient were diluted to a total lipid concentration of 4 mM and mixed with fresh human plasma in a 1:1 volume ratio. Liposomes were incubated in a water bath at 37°C. Samples (100 μL) were then taken out at 0, 1, 3, 6, and 24 hours and placed on Sepharose CL 4B minicolumns (5.5 × 70 mm) pre-equilibrated with 150 mM NaCl solution to separate the liposomal fraction from the released drug (free and protein-bound). The MIT and lipid concentrations were measured in the collected liposomal fractions, and the resulting drug-to-lipid ratio was compared with the initial value.
MIT release from liposomes in acidic pH
AA liposomes were loaded with MIT (drug-to-lipid ratio of 0.2) via ammonium ascorbate and mixed with 200 mM phosphate buffer (pH 5.0 or 7.4) with 0.1% NaN3 in a 1:1 volume ratio and incubated in a water bath at 37°C. The lipid concentration was 2 mM. Samples of 100 μL were then taken out at 0, 1, 3, 6, and 24 hours and placed on Sephadex G-50 Fine mini-columns (5.5 × 70 mm) pre-equilibrated with 150 mM NaCl solution to separate the liposomal fraction from the released drug. CHOL liposomes loaded with MIT (drug-to-lipid ratio of 0.2) via ammonium ascorbate and mixed with 200 mM phosphate buffer (pH 5.0) were treated according to the same procedure as described above. The MIT and lipid concentrations were measured in the collected liposomal fractions, and the resulting drug-to-lipid ratio was compared with the initial value.
Cell culture
Human melanoma cell line A375 (provided by the Laboratory of Cell Pathology, Faculty of Biotechnology, University of Wrocław, Poland) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) culture medium (Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław, Poland) supplemented with 4 mM glutamine (Life Technologies, Carlsbad, CA, USA) and 5% fetal bovine serum (Life Technologies). Human melanoma cell line Hs294T (provided by the Institute of Immunology and Experimental Therapy) was cultured in DMEM culture medium (Lonza, Basel, Switzerland) supplemented with 2 mM glutamine (Life Technologies) and 5% fetal bovine serum (Life Technologies). The normal human dermal fibroblast (NHDF; Lonza) cell line was cultured in MEMα culture medium (Lonza) supplemented with 2 mM glutamine (Life Technologies) and 10% fetal bovine serum (Life Technologies). All the media contained 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 0.25 μg/mL amphotericin B (Lonza). The cells were cultured at 37°C in a humid atmosphere saturated with 5% CO2.
Cytotoxicity
Cytotoxicity was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.31 A375, Hs294T and NHDF cells were seeded into 96-well culture plates at a density of 104 cells per well and incubated at 37°C in a humid atmosphere saturated with 5% CO2. After 24 hours, the medium was changed to serum-free medium that contained serial concentrations of different MIT liposomal formulations (AA and CHOL liposomes with MIT loaded via an ammonium ascorbate gradient, further referred to as vit C AA/CHOL lip MIT; and AA and CHOL liposomes with MIT loaded via an ammonium sulfate gradient, further referred to as AS AA/CHOL lip MIT) or free MIT. The concentration of MIT ranged from 0.2 to 50 μM. Empty CHOL and AA liposomes were added to the wells as a control.
In order to investigate protective effects of ascorbic acid in MIT-treated NHDF cells, three additional formulations were tested: MIT in ascorbic acid solution (concentration similar to the intraliposomal one) and free MIT together with CHOL liposomes loaded with ammonium ascorbate or ammonium sulfate (drug-to-lipid ratio of 0.2).
The MTT test was performed 48 and 72 hours after adding the drug. For this purpose, the medium was carefully removed, and 50 μL of MTT solution was added to each well at a concentration of 0.5 mg/mL in culture medium without serum. Incubation was carried out for 4 hours at 37°C in an atmosphere containing 5% CO2. After this time, the medium with MIT or liposomes was removed, and 50 μL of dimethyl sulfoxide was added to each well. The plate was shaken for about 10 minutes to dissolve formazan crystals resulting from the MTT test. The absorbance was detected using an enzyme-linked immunosorbent assay microplate reader at λ=570 nm.
Statistical analysis
Statistical analysis was performed using the unpaired two-tailed Student’s t-test, with differences considered significant when the P-value was smaller than 0.05.
Results
Liposome characterization
As reported previously,23 it is possible to encapsulate such anthracyclines as epirubicin in liposomes containing 300 mM ammonium ascorbate at pH 4.0. The first aim of our study was to test the possibility of MIT encapsulation in liposomes with a transmembrane gradient of ammonium ascorbate.
It was found that it is possible to encapsulate MIT in CHOL liposomes (HSPC:CHOL:DSPE-PEG2000) with almost 100% efficiency with high (0.2 molar) drug-to-lipid ratios. The addition of 5% mol AA (AA liposomes) did not affect the EE or liposome size (Table 2). The zeta potential did not increase after MIT loading (−1.49±0.53 mV in the case of CHOL liposomes, and −4.5±0.47 mV in the case of AA liposomes; zeta potential before loading is given in Table 2). Higher amounts of AA (from 10% mol up) in the bilayer drastically decreased the EE (data not shown). As a result, all further experiments were focused on liposomes containing 5% AA.
Table 2.
Characteristics of the tested formulations (n=3)
| Formulation | Size (nm) | PDI | Zeta potential (mV) | D/L (molar) | EE (%) |
|---|---|---|---|---|---|
| CHOL liposomes | 112±4 | 0.036±0.004 | −0.87±0.39 | 0.2 | 100.6±2.7 |
| AA liposomes | 112±3 | 0.041±0.003 | −4.31±0.49 | 0.2 | 99.5±3.5 |
Abbreviations: CHOL, cholesterol; AA, anacardic acid; PDI, polydispersity index; D/L, drug-to-lipid ratio; EE, encapsulation efficiency.
Determination of encapsulation efficiency with different drug-to-lipid ratios
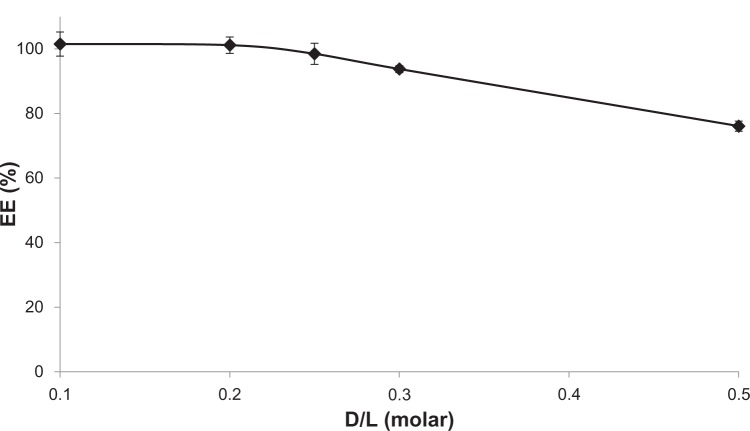
The EE of MIT in AA liposomes was tested. High (>95%) efficiency can be achieved for a drug-to-lipid molar ratio of up to 0.25, and it gradually decreases with increasing ratios. However, even at a drug-to-lipid ratio of 0.5, the majority (over 75%) of the drug can still be encapsulated. The results of the experiment are given in Figure 2.
Figure 2.
Mitoxantrone encapsulation efficiency (EE) in anacardic acid liposomes at different drug-to-lipid (D/L) ratios (molar). The incubation was conducted for 5 minutes at 60°C. n=3.
Determination of the optimal encapsulation temperature of MIT
It is generally acknowledged that the process of anthracycline loading into the liposomes is temperature-dependent, due to the greater bilayer fluidity at the main transition temperature, which facilitates the diffusion of the drug across the membrane. This should be of special importance in the case of MIT, due to the relative hydrophilicity of the drug. The presence of AA in the bilayer could also have an influence on the optimal drug-loading temperature.
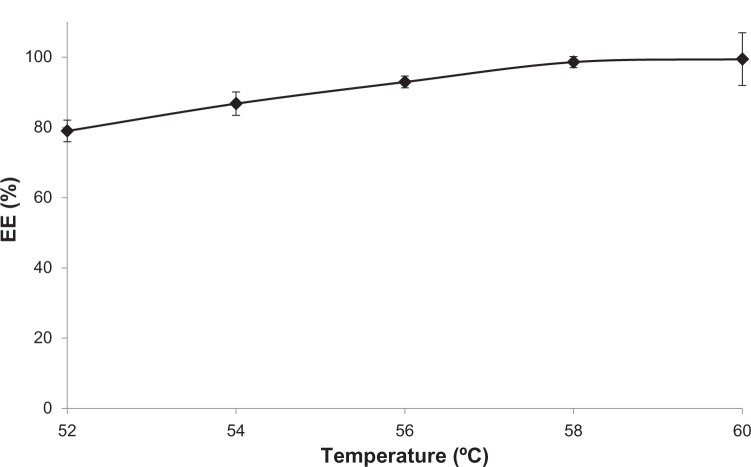
For the purpose of the experiment, MIT was incubated with AA liposomes containing 300 mM ammonium ascorbate (pH 4.0) at a drug-to-lipid molar ratio of 0.2 for 5 minutes. It was found that the EE of MIT in AA liposomes increases with the increase in temperature and reaches >95% at 58°C–60°C (Figure 3), as expected based on the results from epirubicin encapsulation.23 There was no apparent difference in the optimal loading temperature for AA or CHOL liposomes (data not shown).
Figure 3.
The encapsulation efficiency (EE) of mitoxantrone in anacardic acid liposomes depends on the loading temperature. The incubation was conducted for 5 minutes in a drug-to-lipid molar ratio of 0.2. n=3.
Kinetics of MIT encapsulation
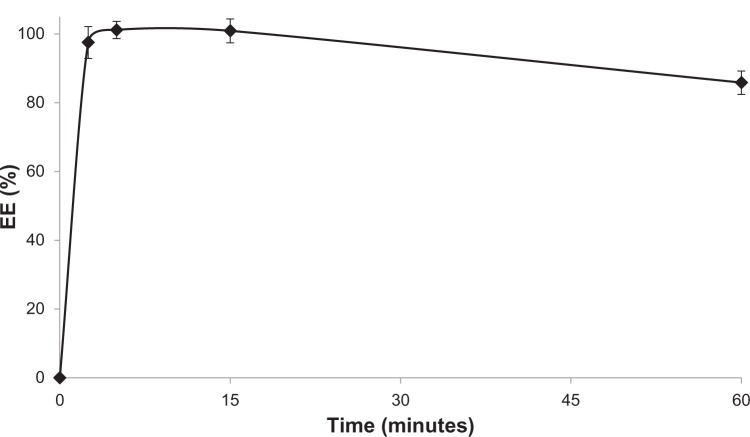
The time required for complete drug loading into AA liposomes was investigated. For the purpose of the experiments, an incubation temperature of 60°C and a drug-to-lipid molar ratio of 0.2 were chosen. Sample aliquots were withdrawn at different time points. No drug was encapsulated at room temperature (time 0). It was found (Figure 4) that the process of encapsulation is extremely rapid, with an EE exceeding 95% after 2.5 minutes. After 1 hour, limited drug leakage (lower than 15%) was observed.
Figure 4.
Kinetics of mitoxantrone encapsulation efficiency (EE) in anacardic acid liposomes. The incubation was conducted at 60°C in a drug-to-lipid molar ratio of 0.2. n=3.
Long-term retention of MIT in AA liposomes – preliminary studies
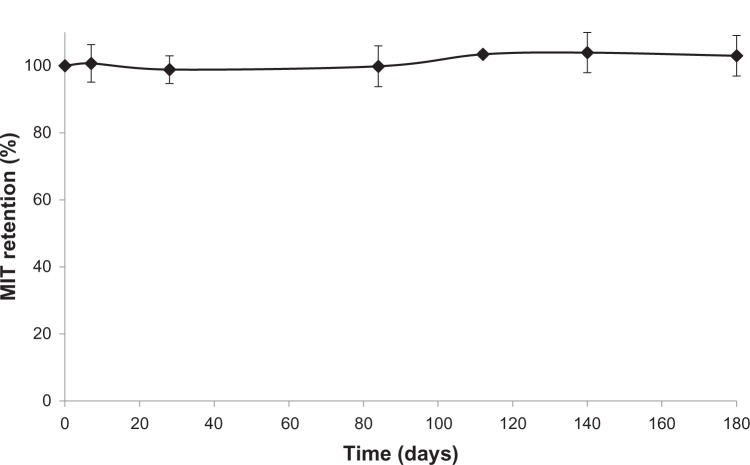
In order to assess preliminarily the long-term stability of liposomal MIT, the drug was incubated with AA liposomes at a drug-to-lipid ratio of 0.2 for 5 minutes at 60°C. Liposomes were then diluted to a lipid concentration of 2 mM and stored at 4°C. At selected time points, aliquots (100 μL) were withdrawn and drug retention was measured. The results show that the formulation is stable in storage for at least 6 months and drug-leakage rate is negligible (Figure 5). After 6 months in storage, the liposome size was 114±1 nm (PDI 0.073±0.011), while at the beginning of the experiment it was 116±2 nm (PDI 0.027±0.006). No apparent liposome aggregation was detected.
Figure 5.
Long-term stability of mitoxantrone (MIT) in anacardic acid liposomes incubated at 4°C – preliminary study. n=3.
MIT release from AA liposomes in the presence of human plasma in vitro
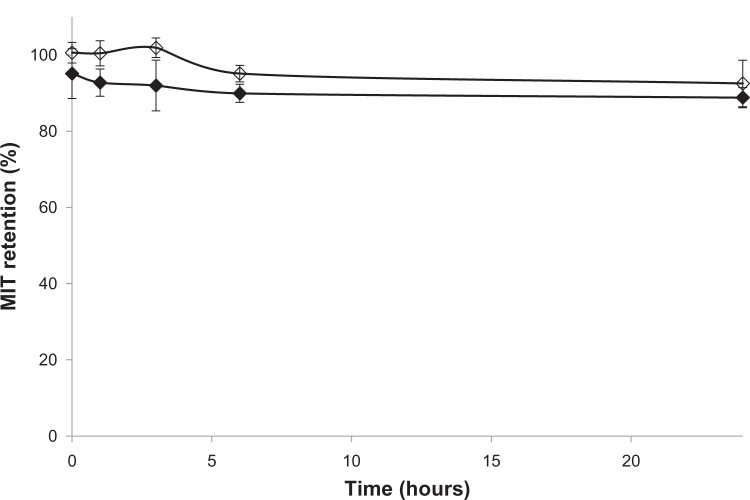
In order to determine the influence of human plasma proteins on the stability of liposomes containing MIT encapsulated by an ammonium ascorbate gradient, CHOL liposomes and AA liposomes with MIT were prepared as described in the Materials and methods section. After 24 hours, marginal drug leakage (<10%) could be observed. The initial release of MIT (<5%) from AA liposomes could be partially due to the presence of the drug on the outer leaflet of the bilayer (Figure 6), where it can be bound electrostatically to negatively charged AA and therefore easily released from the surface by plasma proteins. However, the difference in stability between CHOL and AA liposomes was not significant, so it can be assumed that the presence of AA in the bilayer does not have any negative impact on liposome stability.
Figure 6.
The plasma stability of cholesterol liposomes (⋄) and anacardic acid liposomes (♦) loaded with mitoxantrone (MIT) via an ammonium ascorbate gradient. n=3.
MIT release from AA liposomes in acidic pH
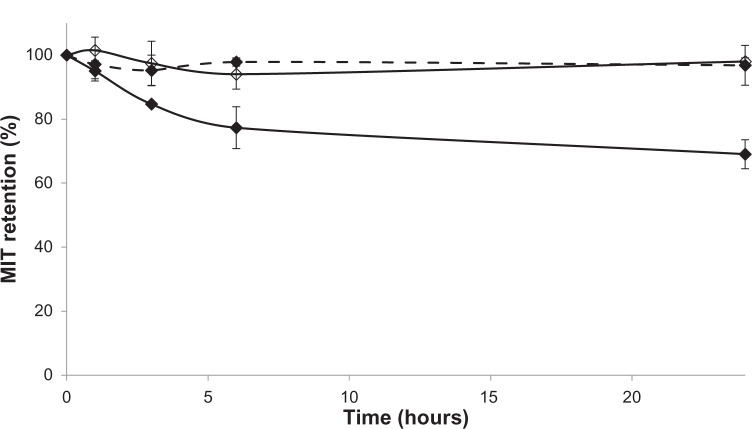
AA is a weak acid with a pKa of 5.8. It is therefore deprotonated and negatively charged under physiological conditions, such as those found in the circulatory system (pH 7.4). At lower pH, such as that found in endosomes, AA becomes uncharged and can traverse the liposomal bilayer by flip-flop movements much more easily. This could contribute to lower stability and as a consequence a faster drug-release rate from these liposomes in acidic pH. To test this hypothesis, MIT was loaded into AA and CHOL liposomes. Then, AA liposomes were incubated at 37°C and pH 5.0 and drug retention was tested. The values were compared with retention in CHOL liposomes incubated at pH 5.0 and AA liposomes incubated at pH 7.4. These three experiments were conducted in parallel (Figure 7).
Figure 7.
Mitoxantrone (MIT) retention in anacardic acid (AA) liposomes (♦) at pH 5.0 (continuous line) and pH 7.4 (dashed line) and in cholesterol liposomes at pH 5.0 (⋄, continuous line). The difference between AA liposomes at pH 5.0 and 7.4 is statistically significant from 3 hours onwards.
After 24 hours’ incubation, the size of AA liposomes at pH 5.0 was 110±1 nm (PDI 0.063±0.025), and at pH 7.4 the size was 112±1 nm (PDI 0.052±0.003). At the beginning of the experiment, their size was 114±2 nm (PDI 0.028±0.018). Therefore, drug release was not a result of liposome aggregation.
Cytotoxicity towards melanoma cell lines
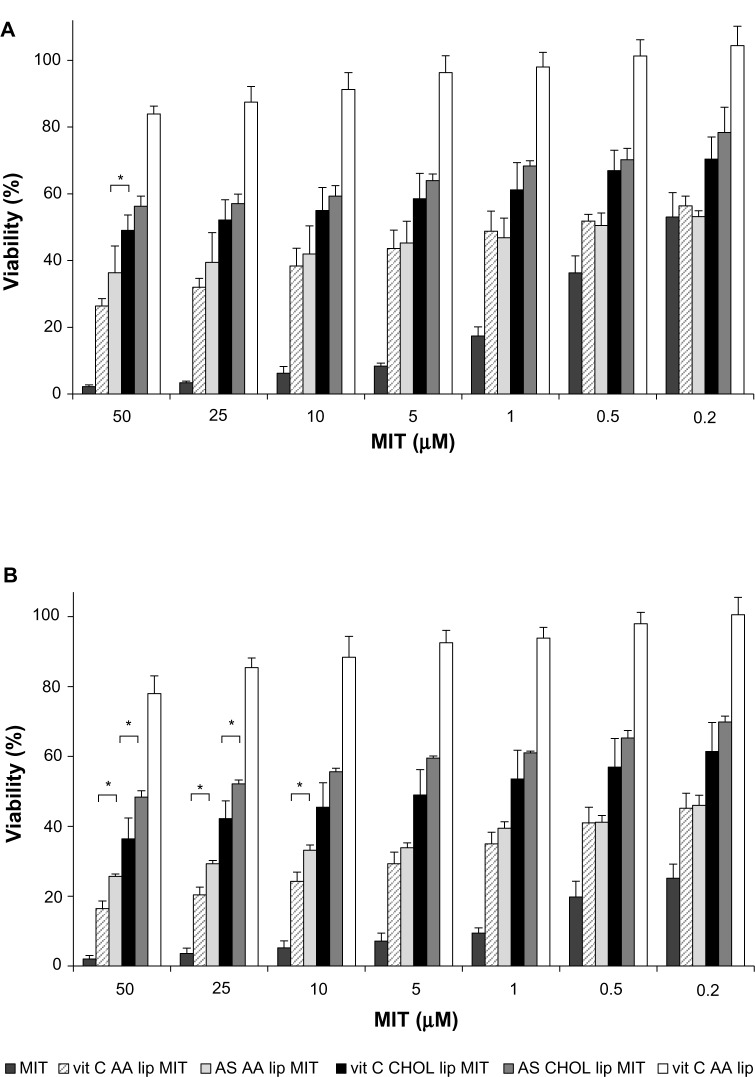
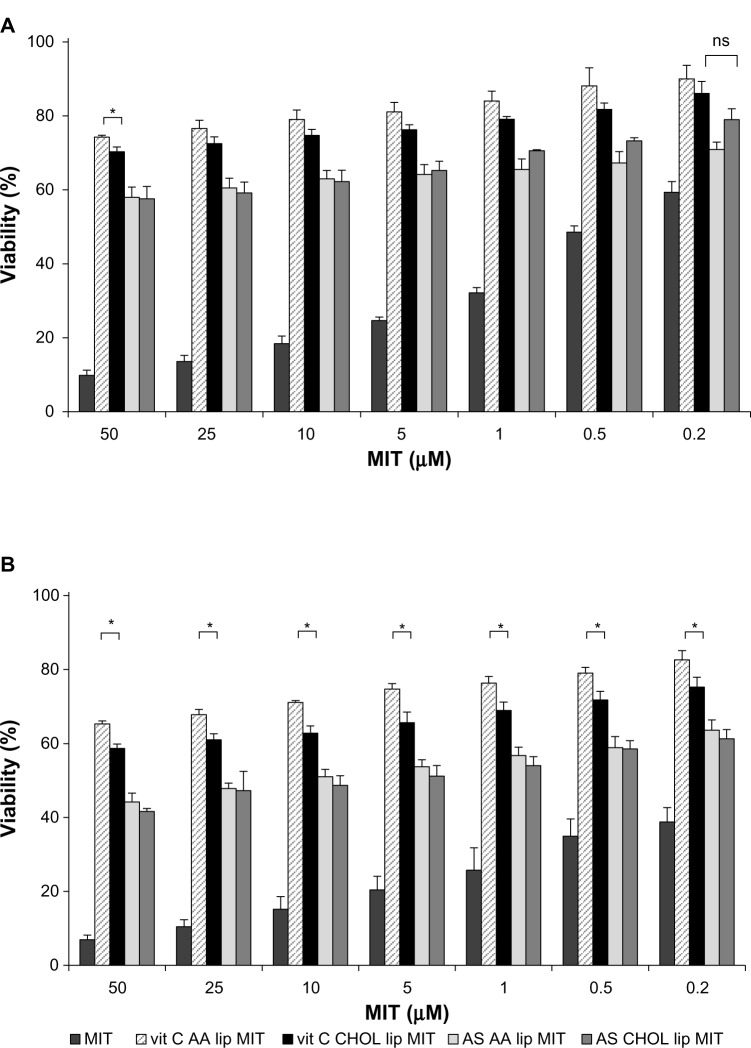
The cytotoxicity of free MIT and liposomal formulations was tested on human melanoma cell lines A375 and Hs294T. The drug was loaded into liposomes via two different gradients: ammonium ascorbate (vit C) and ammonium sulfate (AS). Two lipid compositions were investigated: liposomes with AA (AA lip) and without AA (CHOL lip). The exact composition is given in Table 1. The liposomes were loaded with MIT where indicated. The amount of living cells was measured by MTT assay 48 hours (Figures 8A and 9A) and 72 hours (Figures 8B and 9B) after the addition of the free drug or liposomes.
Figure 8.
Mitoxantrone (MIT) cytotoxicity for the A375 melanoma cell line 48 hours (A) and 72 hours (B) after the addition of the free drug or liposomes. Differences between vitamin C anacardic acid liposome (vit C AA lip) MIT and vit C cholesterol (CHOL) lip MIT and between ammonium sulfate (AS) AA lip MIT and AS CHOL lip MIT are significant for the entire range of MIT concentrations (P<0.05).
Notes: *Other significant differences (P<0.05). Detailed explanation in the text. n.6.
Figure 9.
Mitoxantrone (MIT) cytotoxicity for the Hs294T melanoma cell line 48 hours (A) and 72 hours (panel B) after the addition of the free drug or liposomes. Differences between vitamin C anacardic acid liposome (vit C AA lip) MIT and vit C cholesterol (CHOL) lip MIT and between ammonium sulfate (AS) AA lip MIT and AS CHOL lip MIT are significant for the entire range of MIT concentrations (P<0.05). Detailed explanation in the text. n.6.
Both tested cell lines are sensitive to MIT in a dose-dependent manner to a comparable degree, with Hs294T being less sensitive. Empty AA liposomes (vit C AA lip) show negligible toxicity, which is approximately the same level for both cell lines. The results show that the presence of AA significantly improves cytotoxicity of liposomal MIT loaded via either an ammonium ascorbate or AS gradient. The cytotoxicity of vit C AA lip MIT and AS AA lip MIT is significantly higher than that of vit C CHOL lip MIT and AS CHOL lip MIT, respectively. At higher concentrations and after a longer incubation (72 hours), an additional significant cytotoxic effect of ascorbic acid can be observed, especially with the Hs294T cell line (Figure 9B). The difference in cytotoxicity between vit C AA lip MIT and AS AA lip MIT was statistically significant for the entire range of tested concentrations. The patterns observed for 48-hour incubation remain virtually the same for 72-hour incubation.
Cytotoxicity towards a normal cell line
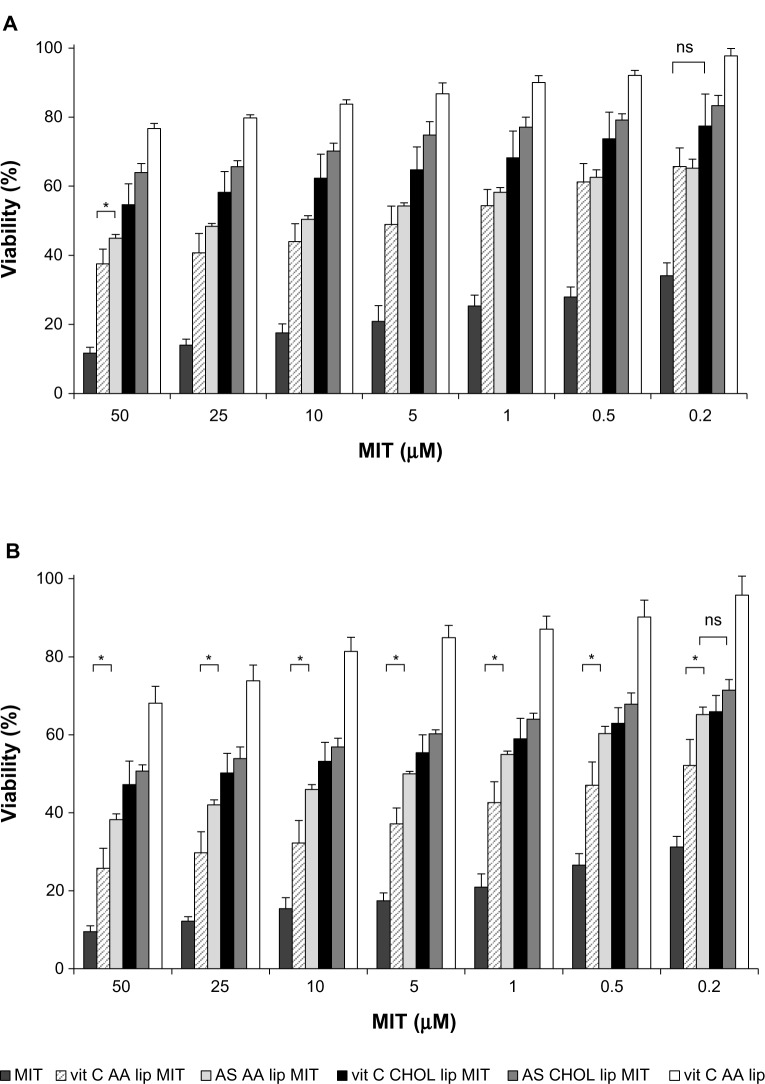
Cytotoxicity towards a normal cell line (NHDF) was assessed for liposomal and free MIT. The drug was loaded into liposomes via two different gradients: vit C and AS. Two lipid compositions were investigated: AA lip and CHOL lip. The exact composition is given in Table 1. The liposomes were loaded with MIT.
The results showed that the cytotoxic activity of liposomal formulations of MIT was lower for formulations loaded with ammonium ascorbate (vit C AA lip MIT and vit C CHOL lip MIT) than for those loaded with AS (AS AA lip MIT and AS CHOL lip MIT). The differences were statistically significant for the entire concentration range, suggesting a protective effect of vitamin C on NHDF. After longer incubation (72 hours), the formulation with AA (vit C AA lip MIT) showed a significantly decreased toxicity compared to the formulation without AA (vit C CHOL lip MIT), suggesting an additional protective mechanism involving AA.
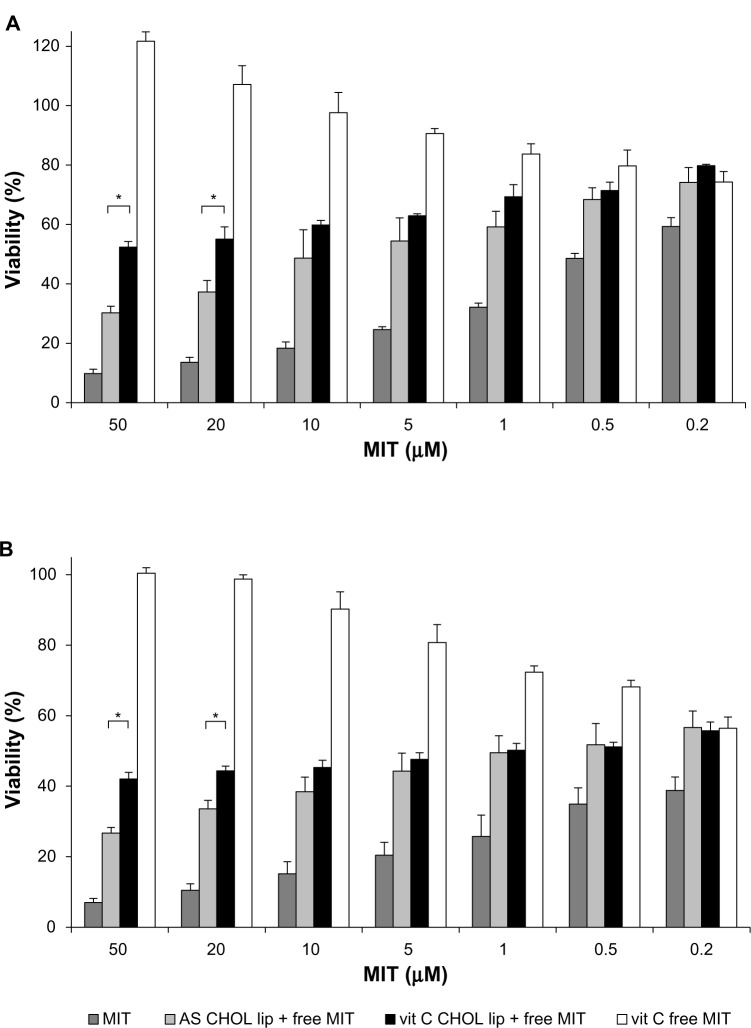
Additional experiments were conducted to confirm the findings suggesting a protective role of vitamin C on NHDF treated with MIT. Cells were incubated with CHOL liposomes loaded with ammonium ascorbate mixed with free MIT (vit C CHOL lip + free MIT), CHOL liposomes loaded with AS mixed with free MIT (AS CHOL lip + free MIT) and free MIT mixed with ammonium ascorbate (vit C free MIT) in a ratio resembling the MIT:vit C ratio inside the liposomes (Figure 10). Cell viability was assessed using the MTT assay 48 hours (Figure 11A) and 72 hours (Figure 11B) after the addition of the drug.
Figure 10.
Mitoxantrone (MIT) cytotoxicity for the normal human dermal fibroblast cell line 48 hours (A) and 72 hours (B) after the addition of the free drug or liposomes. Differences between vitamin C anacardic acid liposome (vit C AA lip) and ammonium sulfate (AS) AA lip MIT and between vit C cholesterol (CHOL) lip MIT and AS CHOL lip MIT are significant for the entire range of MIT concentrations, unless indicated otherwise (P<0.05).
Notes: *Other significant differences (P<0.05). Detailed explanation in the text. n=6.
Abbreviation: ns, not significant.
Figure 11.
Mitoxantrone (MIT) cytotoxicity for the normal human dermal fibroblast cell line 48 hours (A) and 72 hours (B) after the addition of the free drug or liposomes with the free drug.
Notes: *Significant differences (P<0.05). Detailed explanation in the text. n.3.
Abbreviations: vit, vitamin; lip, liposomes; AS, ammonium sulfate; CHOL, cholesterol.
The formulations resulting from mixing free MIT with liposomes (vit C CHOL lip + free MIT, AS CHOL lip + free MIT) were more cytotoxic than the formulations where MIT was encapsulated in the liposomes (vit C CHOL lip MIT, AS CHOL lip MIT). This could be expected based on the fact that free MIT was more cytotoxic than MIT encapsulated in liposomes in all the conducted experiments. At higher concentrations, the toxicity of MIT mixed with vit C CHOL liposomes was significantly lower than the toxicity of MIT mixed with AS CHOL liposomes. The cytotoxic activity was abolished by mixing MIT with high concentration of ammonium ascorbate (vit C free MIT), which confirms the findings regarding the protective role of vitamin C.
Discussion
In this study, we investigated the antineoplastic activity of liposomal MIT loaded via a vitamin C gradient developed in our laboratory23 coencapsulated with AA for improved anticancer activity. The method of AA isolation and purification was also developed in our laboratory.
The liposomal formulation of MIT with AA was characterized in terms of basic parameters, such as size and zeta potential, optimal loading time, temperature, and drug-to-lipid ratio. Storage stability at 4°C was studied preliminarily, as was drug release in the presence of human plasma in vitro and at acidic pH. In vitro toxicity studies were conducted using melanoma and normal cell lines.
The ammonium ascorbate gradient proved to be an efficient method for MIT remote loading, as it resulted in high EEs for a drug-to-lipid ratio of up to 0.25. Even at a drug-to-lipid ratio of 0.5, the EE exceeded 75% (Figure 2). The loading process was temperature-dependent: the drug could not be encapsulated in room temperature. After 5 minutes’ incubation, the EE rose from 80% at 52°C to 100% at 60°C (Figure 3). MIT has relatively low hydrophobic properties, so it does not easily permeate the bilayer. The bilayer needs to be heated to a point over the main transition temperature to become more fluid and permeable.
The preliminary studies confirm the stability of MIT encapsulated in liposomes containing AA. After 6 months of storage at 4°C, no drug release was observed (Figure 5). No apparent changes in liposome size or PDI were observed after that period, suggesting that no liposome aggregation occurred.
The presence of AA in the bilayer did not have any significant effect on drug retention in 50% human plasma in vitro (Figure 6). Drug retention was around 90% in the case of liposomes with or without AA loaded with ammonium ascorbate. The initial release of the drug from AA liposomes (<5%) was not observed for CHOL liposomes, but could have partially been due to a small amount of the drug being electrostatically bound to negatively charged AA on the outer leaflet of the liposomes. After mixing with the human plasma, those drug molecules were immediately captured by plasma proteins. However, after that initial release, there was no significant further release observed in the remaining 24 hours, suggesting good pharmacokinetic parameters for the investigated formulation.
Currently, novel drug-delivery systems tend to serve more than one function.32 The proposed AA liposomes loaded with ammonium ascorbate and MIT could enhance drug intracellular delivery, apart from having the widely acknowledged properties of liposomes, namely a prolonged circulation time and tumor tissue targeting thanks to the enhanced permeability-and-retention effect. As discussed before, AA has a pKa of 5.8, so it is uncharged in the inner leaflet of liposomes (pH 4.0) and negatively charged in the outer leaflet (pH 7.4). It was reported previously that charged fatty acids could not traverse the bilayer by flip-flop movements.33 Therefore, under physiological conditions, only one-way movement is possible for AA. However, when the external pH becomes acidified, such as in endosomes, AA becomes uncharged in the outer leaflet and can more easily traverse the bilayer. This could contribute to the decreased stability of AA liposomes at pH 5.0 and the observed drug release of up to 30% after 24 hours’ incubation (Figure 7). This phenomenon is unlikely to be the result of the release of electrostatically bound drug to the outer surface of liposomes, because if such an amount of drug was bound electrostatically, then drug encapsulation at room temperature would have been higher than 0. The loss of charge on AA in the outer bilayer could also influence membrane curvature and induce defects that promote drug release, as seen in the case of other anionic pH-sensitive liposomes.34 AA was also found to dissipate transmembrane gradients,35 which could contribute to drug release at acidic pH.
The in vitro cytotoxicity towards melanoma cell lines showed that AA significantly increased the activity of liposomal mitoxantrone at all of the tested concentrations, no matter which gradient was used. One explanation could be the improved intracellular delivery of the drug, as discussed earlier. However, the release rate seems too slow for this to be the only factor contributing to such an increase in cytotoxicity. A possible synergism between AA and MIT could be more important. It should be noted that AA liposomes alone, without MIT, showed only slight inhibition of melanoma cell-line proliferation.
AA is a commonly acknowledged antineoplastic molecule that induces caspase-independent apoptosis36 and inhibits the NF-κB pathway.3 It is also an epigenetic agent that inhibits histone acetyltransferases (HATs) involved in the regulation of gene expression. One of the proteins from the HAT family, Tip60, is involved in the cell response to genotoxic events, such as strand breaks.37 Inhibiting that enzyme impairs DNA-repair systems. AA was found to sensitize cancer cells to genotoxic damage via Tip60 inhibition.38 It is known that one of the main mechanisms of action of MIT is to induce DNA damage, and thus AA-mediated impairment of the repair system could contribute to the observed increase in toxicity.
Using an ascorbic acid gradient enhanced cytotoxicity even further when compared with AS-loaded liposomes. Vitamin C was found to improve the activity of several anthracyclines, most notably doxorubicin22 by enhancing the ROS-mediated DNA damage caused by that anthracycline. In the case of mitoxantrone, the formation of ROS is limited, so other mechanisms of improved cytotoxicity of MIT loaded via an ammonium ascorbate gradient are more probable. This could be caused by vitamin C-mediated p53 upregulation,39 inhibition of cyclooxygenase-2 expression,40 and induction of apoptosis, which was demonstrated for the A375 melanoma cell line.20
A distinctly different activity of ascorbic acid was observed with normal fibroblasts (NHDF). The formulations containing MIT loaded via an ammonium ascorbate gradient were significantly less cytotoxic than the formulations with MIT loaded using an AS gradient. It has been reported that ascorbic acid could play a role in cell protection against genotoxic damage, but the exact mechanism of protection remained unclear.21 The major postulated mechanism of ascorbic acid protective activity is its role in scavenging ROS,41 but this is unlikely to be the main factor that contributes to NHDF protection against MIT, because MIT-mediated ROS production is limited. Duarte et al42 recently performed gene-expression profiling in human skin cells treated with ascorbic acid, and found out that such treatment resulted in an increase in the expression of genes involved in cell proliferation and DNA repair. Another study showed that vitamin C could stimulate chemical repair of the DNA.43 This could explain the ROS-independent protective mechanism of ascorbic acid in NHDF.
In the case of MIT encapsulated in CHOL liposomes via either gradient and MIT added together with CHOL liposomes (Figures 10 and 11), an apparent difference in cytotoxicity could be observed between these two sets of formulations. The latter were more cytotoxic because the drug could freely and independently diffuse across the cell membrane, while the former needed to undergo endocytosis before the drug could exert its intracellular effect.
AA liposomes loaded with MIT via an ammonium ascorbate gradient were significantly less cytotoxic towards NHDF than CHOL liposomes loaded with MIT via this gradient (Figure 10B). This may be the result of p300 HAT inhibition by AA. It was shown that cellular stress could lead to matrix metalloproteinase-1 (MMP-1) induction and result in effects such as double-strand breaks of the DNA in human skin fibroblasts.44 The same study demonstrated that AA successfully inhibited MMP-1 gene expression and subsequent apoptosis by inhibiting p300 HAT. We suppose that a similar mechanism plays a role in AA-mediated protection of NHDF against the cytotoxic activity of MIT.
Treating cells with MIT combined with a high concentration of ascorbic acid (Figure 11) resulted in a pronounced abolition of MIT toxicity. Apart from the protective mechanisms discussed earlier, this could be a result of the antagonistic effect of ascorbic acid at high concentrations, which was observed in the case of some anticancer drugs.45 However, the effect of ascorbic acid on neoplastic and normal cell lines and its synergism/antagonism with other drugs remains ambiguous. Further studies are required to elucidate the exact molecular mechanisms of enhancing MIT toxicity in melanoma cell lines while decreasing it in normal skin fibroblasts.
Our new liposomal formulation of MIT is stable and efficient in terms of drug loading. It enhances drug release in acidic pH, and combines a cytotoxic drug with a possibly synergistic epigenetic agent – AA. Using a vitamin C gradient enhances the cytotoxic activity of the formulation towards cancer cells, and it plays a protective role in the case of normal cells. All these factors combined make it a potentially clinically relevant formulation. This should be confirmed further using in vivo studies.
Conclusion
We have presented the results of a study on a novel liposomal formulation of MIT with AA. Remote loading of MIT driven by an ammonium ascorbate gradient allowed a high amount of drug (drug-to-lipid ratio of up to 0.3, with an EE of >90%) to be loaded in less than 5 minutes. The formulation was found to be stable in storage (no apparent release after 6 months at 4°C) and in human plasma (retention over 90% after 24 hours’ incubation). The liposomes are pH-sensitive, with 30% of the drug being released at pH 5.0 after 24 hours’ incubation. The addition of AA enhanced the cytotoxicity of the formulation towards melanoma cell lines. Liposomes with MIT loaded via an ammonium ascorbate gradient proved to be more cytotoxic towards melanoma cell lines at high (10 μM and higher) MIT concentrations than the corresponding liposomes with MIT loaded via an AS gradient. The cytotoxicity studies on NHDF cells demonstrated that vitamin C played a cytoprotective role, decreasing MIT-induced cytotoxicity when compared with liposomes containing AS. All these factors contribute to the possible clinical relevance of MIT loaded via ammonium ascorbate into AA liposomes, but in vivo studies are required in order to confirm these findings.
Acknowledgments
The authors would like to thank the University of Wrocław for its support of this research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. Small molecule modulators of histone acetyltransferase p300. J Biol Chem. 2003;278(21):19134–19140. doi: 10.1074/jbc.M301580200. [DOI] [PubMed] [Google Scholar]
- 2.Rajendran P, Ho E, Williams DE, Dashwood RH. Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells. Clin Epigenetics. 2011;3(1):4. doi: 10.1186/1868-7083-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung B, Pandey MK, Ahn KS, et al. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood. 2008;111(10):4880–4891. doi: 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, He L, Zhang L, et al. Anacardic acid (6-pentadecylsalicylic acid) inhibits tumor angiogenesis by targeting Src/FAK/Rho GTPases signaling pathway. J Pharmacol Exp Ther. 2011;339(2):403–411. doi: 10.1124/jpet.111.181891. [DOI] [PubMed] [Google Scholar]
- 5.Tamura S, Nitoda T, Kubo I. Effects of salicylic acid on mushroom tyrosinase and B16 melanoma cells. Z Naturforsch C. 2007;62(3–4):227–233. doi: 10.1515/znc-2007-3-412. [DOI] [PubMed] [Google Scholar]
- 6.Park WJ, Ma E. Inhibition of PCAF histone acetyltransferase, cytotoxicity and cell permeability of 2-acylamino-1-(3- or 4-carboxy-phenyl) benzamides. Molecules. 2012;17(11):13116–13131. doi: 10.3390/molecules171113116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan J, Chen B, He L, et al. Anacardic acid (6-pentadecylsalicylic acid) induces apoptosis of prostate cancer cells through inhibition of androgen receptor and activation of p53 signaling. Chin J Cancer Res. 2012;24(4):275–283. doi: 10.3978/j.issn.1000-9604.2012.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz DJ, Wickramasinghe NS, Ivanova MM, et al. Anacardic acid inhibits estrogen receptor alpha-DNA binding and reduces target gene transcription and breast cancer cell proliferation. Mol Cancer Ther. 2010;9(3):594–605. doi: 10.1158/1535-7163.MCT-09-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RK, Zee-Cheng RK, Lee WW, Acton EM, Henry DW, Cheng CC. Experimental antitumor activity of aminoanthraquinones. Cancer Treat Rep. 1979;63(3):425–439. [PubMed] [Google Scholar]
- 10.Novak RF, Kharasch ED. Mitoxantrone: propensity for free radical formation and lipid peroxidation – implications for cardiotoxicity. Invest New Drugs. 1985;3(2):95–99. doi: 10.1007/BF00174155. [DOI] [PubMed] [Google Scholar]
- 11.Rossato LG, Costa VM, de Pinho PG, et al. The metabolic profile of mitoxantrone and its relation with mitoxantrone-induced cardiotoxicity. Arch Toxicol. 2013;87(10):1809–1820. doi: 10.1007/s00204-013-1040-6. [DOI] [PubMed] [Google Scholar]
- 12.Martinelli V, Radaelli M, Straffi L, Rodegher M, Comi G. Mitoxantrone: benefits and risks in multiple sclerosis patients. Neurol Sci. 2009;30(Suppl 2):S167–S170. doi: 10.1007/s10072-009-0142-7. [DOI] [PubMed] [Google Scholar]
- 13.Foye WO, Vajragupta O, Sengupta SK. DNA-binding specificity and RNA polymerase inhibitory activity of bis(aminoalkyl)anthraquinones and bis(methylthio)vinylquinolinium iodides. J Pharm Sci. 1982;71(2):253–257. doi: 10.1002/jps.2600710228. [DOI] [PubMed] [Google Scholar]
- 14.De Isabella P, Palumbo M, Sissi C, et al. Topoisomerase II DNA cleavage stimulation, DNA binding activity, cytotoxicity, and physico-chemical properties of 2-aza- and 2-aza-oxide-anthracenedione derivatives. Mol Pharmacol. 1995;48(1):30–38. [PubMed] [Google Scholar]
- 15.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17(5):421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evenson DP, Darzynkiewicz Z, Staiano-Coico L, Traganos F, Melamed MR. Effects of 9,10-anthracenedione, 1,4-bis[(2-[(2-hydroxyethyl)amino]-ethyl)amino]-, diacetate on cell survival and cell cycle progression in cultured mammalian cells. Cancer Res. 1979;39(7 Pt 1):2574–2581. [PubMed] [Google Scholar]
- 17.Shenkenberg TD, Von Hoff DD. Mitoxantrone: a new anticancer drug with significant clinical activity. Ann Intern Med. 1986;105(1):67–81. doi: 10.7326/0003-4819-105-1-67. [DOI] [PubMed] [Google Scholar]
- 18.Amato RJ, Saxena S, Stepankiw M. Phase II trial assessing granulocyte-macrophage-colony stimulating factor, ketoconazole plus mitoxantrone in metastatic castration-resistant prostate cancer progressing after docetaxel treatments. Cancer Invest. 2013;31(3):177–182. doi: 10.3109/07357907.2013.764564. [DOI] [PubMed] [Google Scholar]
- 19.Huber R, Krüger I, Kuper K, Huber PM, Pichlmaier H. Isolated hyperthermic perfusion with mitoxantrone of melphalan in malignant melanoma of the limb. Am J Surg. 1995;170(4):345–352. doi: 10.1016/s0002-9610(99)80301-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin SY, Lai WW, Chou CC, et al. Sodium ascorbate inhibits growth via the induction of cell cycle arrest and apoptosis in human malignant melanoma A375.S2 cells. Melanoma Re. 2006;16(6):509–519. doi: 10.1097/01.cmr.0000232297.99160.9e. [DOI] [PubMed] [Google Scholar]
- 21.Türkez H, Aydin E. The protective role of ascorbic acid on imazalil-induced genetic damage assessed by the cytogenetic tests. Toxicol Ind Health. 2012;28(7):648–654. doi: 10.1177/0748233711420471. [DOI] [PubMed] [Google Scholar]
- 22.Kurbacher CM, Wagner U, Kolster B, Andreotti PE, Krebs D, Bruckner HW. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett. 1996;103(2):183–189. doi: 10.1016/0304-3835(96)04212-7. [DOI] [PubMed] [Google Scholar]
- 23.Lipka D, Gubernator J, Filipczak N, et al. Vitamin C-driven epirubicin loading into liposomes. Int J Nanomedicine. 2013;8(1):3573–3585. doi: 10.2147/IJN.S47745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakakibara T, Chen FA, Kida H, et al. Doxorubicin encapsulated in sterically stabilized liposomes is superior to free drug or drug-containing conventional liposomes at suppressing growth and metastases of human lung tumor xenografts. Cancer Res. 1996;56(16):3743–3746. [PubMed] [Google Scholar]
- 25.Li C, Cui J, Li Y, et al. Copper ion-mediated liposomal encapsulation of mitoxantrone: the role of anions in drug loading, retention and release. Eur J Pharm Sci. 2008;34(4–5):333–344. doi: 10.1016/j.ejps.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Cui J, Li C, Wang L, et al. Ni2+-mediated mitoxantrone encapsulation: improved efficacy of fast release formulation. Int J Pharm. 2009;368(1–2):24–30. doi: 10.1016/j.ijpharm.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Cui J, Wang C, et al. Lipid composition and grafted PEG affect in vivo activity of liposomal mitoxantrone. Int J Pharm. 2008;362(1–2):60–66. doi: 10.1016/j.ijpharm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Olson F, Hunt CA, Szoka FC, Vail WJ, Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochimica et biophysica acta. 1979;557(1):9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- 29.Chong CS, Colbow K. Light scattering and turbidity measurements on lipid vesicles. Biochim Biophys Acta. 1976;436(2):260–282. doi: 10.1016/0005-2736(76)90192-9. [DOI] [PubMed] [Google Scholar]
- 30.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104(1):10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.Torchilin VP. Nanocarriers. Pharm Res. 2007;24(12):2333–2334. doi: 10.1007/s11095-007-9463-5. [DOI] [PubMed] [Google Scholar]
- 33.Jezek P, Modriansky M, Garlid KD. Inactive fatty acids are unable to flip-flop across the lipid bilayer. FEBS Lett. 1997;408(2):161–165. doi: 10.1016/s0014-5793(97)00334-7. [DOI] [PubMed] [Google Scholar]
- 34.Fattal E, Couvreur P, Dubernet C. “Smart” delivery of antisense oligonucleotides by anionic pH-sensitive liposomes. Adv Drug Deliv Rev. 2004;56(7):931–946. doi: 10.1016/j.addr.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Toyomizu M, Okamoto K, Akiba Y, Nakatsu T, Konishi T. Anacardic acid-mediated changes in membrane potential and pH gradient across liposomal membranes. Biochim Biophys Acta. 2002;1558(1):54–62. doi: 10.1016/s0005-2736(01)00422-9. [DOI] [PubMed] [Google Scholar]
- 36.Seong YA, Shin PG, Kim GD. Anacardic acid induces mitochondrial- mediated apoptosis in the A549 human lung adenocarcinoma cells. Int J Oncol. 2013;42(3):1045–1051. doi: 10.3892/ijo.2013.1763. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A. 2005;102(37):13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Jiang X, Chen S, Price BD. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 2006;580(18):4353–4356. doi: 10.1016/j.febslet.2006.06.092. [DOI] [PubMed] [Google Scholar]
- 39.An SH, Kang JH, Kim DH, Lee MS. Vitamin C increases the apoptosis via up-regulation p53 during cisplatin treatment in human colon cancer cells. BMB Rep. 2011;44(3):211–216. doi: 10.5483/BMBRep.2011.44.3.211. [DOI] [PubMed] [Google Scholar]
- 40.Lee SK, Kang JS, Jung da J, et al. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008;216(1):180–188. doi: 10.1002/jcp.21391. [DOI] [PubMed] [Google Scholar]
- 41.Eguchi M, Miyazaki T, Masatsuji-Kato E, Tsuzuki T, Oribe T, Miwa N. Cytoprotection against ischemia-induced DNA cleavages and cell injuries in the rat liver by pro-vitamin C via hydrolytic conversion into ascorbate. Mol Cell Biochem. 2003;252(1–2):17–23. doi: 10.1023/a:1025567400384. [DOI] [PubMed] [Google Scholar]
- 42.Duarte TL, Cooke MS, Jones GD. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic Biol Med. 2009;46(1):78–87. doi: 10.1016/j.freeradbiomed.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 43.Hata K, Urushibara A, Yamashita S, Shikazono N, Yokoya A, Katsumura Y. Chemical repair of base lesions, AP-sites, and strand breaks on plasmid DNA in dilute aqueous solution by ascorbic acid. Biochem Biophys Res Commun. 2013;434(2):341–345. doi: 10.1016/j.bbrc.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 44.Kim MK, Shin JM, Eun HC, Chung JH. The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PloS One. 2009;4(3):e4864. doi: 10.1371/journal.pone.0004864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heaney ML, Gardner JR, Karasavvas N, et al. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 2008;68(19):8031–8038. doi: 10.1158/0008-5472.CAN-08-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]