Abstract
We have detected seemingly uninduced interferons (IFNs) in 29/37 human placental samples obtained during caesarian sections at different periods of pregnancy, mostly around the 37th week. The amounts were usually low and did not enable us to correlate our findings with any physiological or pathological conditions. Occasionally the presence of IFN was masked by a lectin-like antagonist. Therefore, in a number of cases, substantially higher amounts of IFN were found after purification by affinity chromatography using concanavalin A, Cibacron blue, or antiserum to IFN-alpha, each coupled to Sepharose. Analysis by sodium dodecyl sulfate/polyacrylamide gel electrophoresis revealed the presence of IFN-alpha and IFN-beta with molecular masses between 15 and 80 kilodaltons. Some of the high molecular weight components were neutralized either only by monospecific antiserum to IFN-alpha or, to the same extent, by antiserum to IFN-alpha or to IFN-beta, reminiscent of those previously reported after viral induction in the human amniotic membrane. We postulate that both IFNs and antagonist play a physiological role during fetal development.
Full text
PDF

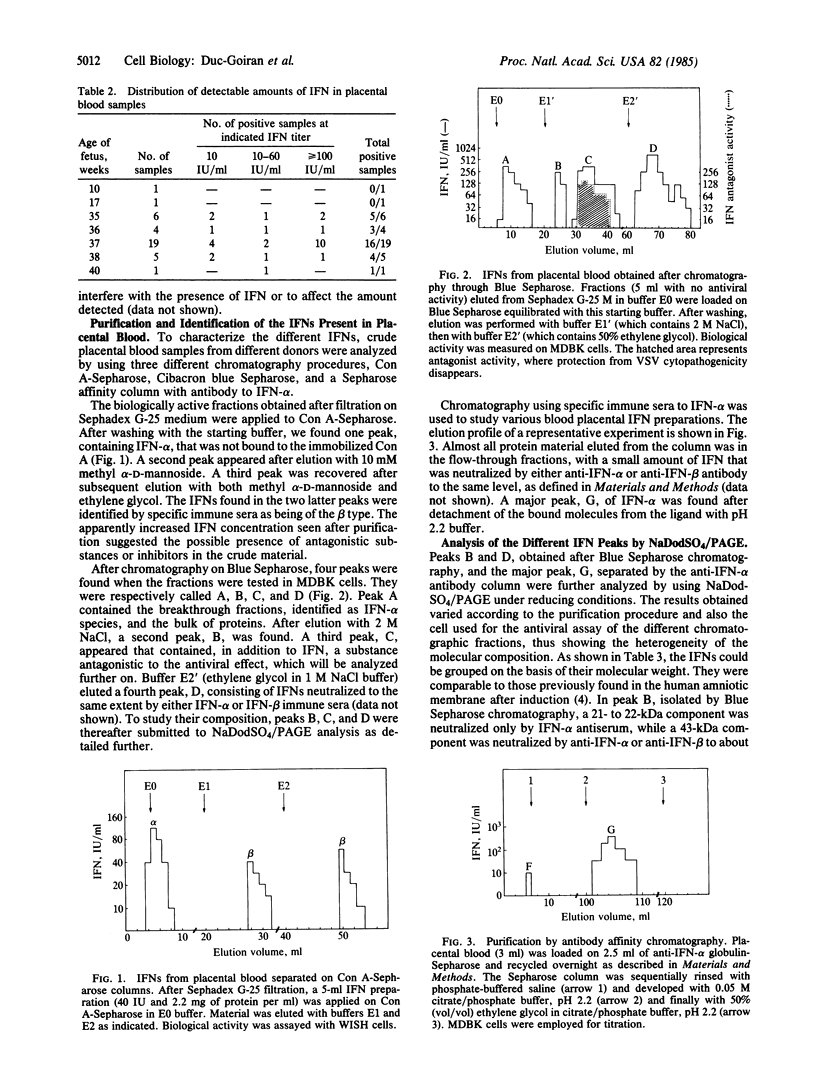
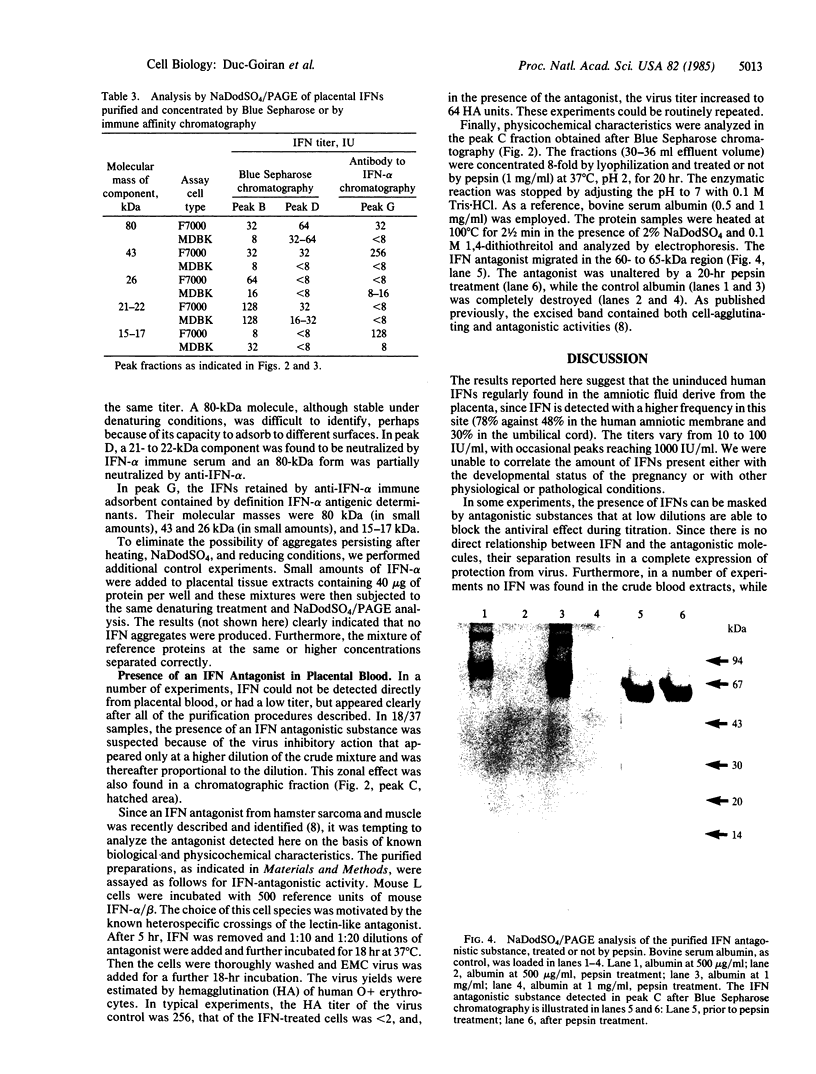

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg K., Ogburn C. A., Paucker K., Mogensen K. E., Cantell K. Affinity chromatography of human leukocyte and diploid cell interferons on sepharose-bound antibodies. J Immunol. 1975 Feb;114(2 Pt 1):640–644. [PubMed] [Google Scholar]
- Bourgeade M. F., Rousset S., Paulin D., Chany C. Reorganization of the cytoskeleton by interferon in MSV-transformed cells. J Interferon Res. 1981 Feb;1(2):323–332. doi: 10.1089/jir.1981.1.323. [DOI] [PubMed] [Google Scholar]
- Chany-Fournier F. Rôle de l'interféron dans la réversion phénotypique des cellules transformées: perte du caractère de malignité. Pathol Biol (Paris) 1983 Mar;31(3):199–213. [PubMed] [Google Scholar]
- Chany C., Lemaître J., Grégoire A. Facteurs antagonistes de l'action antivirale de l'interféron extraites de différentes sarcomes humains. C R Acad Sci Hebd Seances Acad Sci D. 1969 Dec 22;269(25):2628–2630. [PubMed] [Google Scholar]
- Davey M. W., Huang J. W., Sulkowski E., Carter W. A. Hydrophobic interaction of human interferon with concanavalin A-agarose. J Biol Chem. 1974 Oct 10;249(19):6354–6355. [PubMed] [Google Scholar]
- Davey M. W., Sulkowski E., Carter W. A. Binding of human fibroblast interferon to concanavalin A-agarose. Involvement of carbohydrate recognition and hydrophobic interaction. Biochemistry. 1976 Feb 10;15(3):704–713. doi: 10.1021/bi00648a039. [DOI] [PubMed] [Google Scholar]
- Davis L. E., McLaren L. C., Stewart J. A., James C. G., Levine M. D., Skipper B. J. Immunological and microbiological studies of midtrimester amniotic fluid. Gynecol Obstet Invest. 1983;16(5):261–268. doi: 10.1159/000299275. [DOI] [PubMed] [Google Scholar]
- Dirksen E. R., Levy J. A. Virus-like particles in placentas from normal individuals and patients with systemic lupus erythematosus. J Natl Cancer Inst. 1977 Oct;59(4):1187–1192. doi: 10.1093/jnci/59.4.1187. [DOI] [PubMed] [Google Scholar]
- Duc-Goiran P., Robert-Galliot B., Chudzio T., Chany C. Unusual human interferons produced by virus-infected amniotic membranes. Proc Natl Acad Sci U S A. 1983 May;80(9):2628–2631. doi: 10.1073/pnas.80.9.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. K., Reed C. D., Giron D. J. Identification of an interferon in murine placentas. Nature. 1980 Jul 17;286(5770):266–267. doi: 10.1038/286266a0. [DOI] [PubMed] [Google Scholar]
- Gerfaux J., Rousset S., Chany-Fournier F., Chany C. Interferon effect on collagen and fibronectin distribution in the extracellular matrix of murine sarcoma virus-transformed cells. Cancer Res. 1981 Sep;41(9 Pt 1):3629–3634. [PubMed] [Google Scholar]
- Jankowski W. J., von Muenchhausen W., Sulkowski E., Carter W. A. Binding of human interferons to immobolized Cibacron Blue F3GA: The nature of molecular interaction. Biochemistry. 1976 Nov 16;15(23):5182–5187. doi: 10.1021/bi00668a036. [DOI] [PubMed] [Google Scholar]
- Jiang P. H., Chany-Fournier F., Robert-Galliot B., Sarragne M., Chany C. Sarcolectin: an interferon antagonist extracted from hamster sarcomas and normal muscles. Isolation, characterization, and purification. J Biol Chem. 1983 Oct 25;258(20):12361–12367. [PubMed] [Google Scholar]
- Kawade Y. An analysis of neutralization reaction of interferon by antibody: a proposal on the expression of neutralization titer. J Interferon Res. 1980 Fall;1(1):61–70. doi: 10.1089/jir.1980.1.61. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebon P., Girard S., Thépot F., Chany C. The presence of alpha-interferon in human amniotic fluid. J Gen Virol. 1982 Apr;59(Pt 2):393–396. doi: 10.1099/0022-1317-59-2-393. [DOI] [PubMed] [Google Scholar]
- PAUCKER K., CANTELL K., HENLE W. Quantitative studies on viral interference in suspended L cells. III. Effect of interfering viruses and interferon on the growth rate of cells. Virology. 1962 Jun;17:324–334. doi: 10.1016/0042-6822(62)90123-x. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Wang E., Tamm I. Interferon effects on microfilament organization, cellular fibronectin distribution, and cell motility in human fibroblasts. J Cell Biol. 1980 Apr;85(1):9–17. doi: 10.1083/jcb.85.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyhälä Neutralizing antibodies against human leukocyte, lymphoblastoid and fibroblast interferons elicited by immunization with human leukocyte interferon. Acta Pathol Microbiol Scand C. 1978 Dec;86C(6):291–298. doi: 10.1111/j.1699-0463.1978.tb02593.x. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., de Maeyer-Guignard J., Fauconnier B., de Maeyer E. Purification of mouse interferon by affinity chromatography on a solid-phase immunoadsorbent. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1037–1040. doi: 10.1073/pnas.70.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Smith M. E., Bridgen P. J., zur Nedden D., Anfinsen C. B. Purification and partial characterization of human lymphoblast interferon. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5601–5605. doi: 10.1073/pnas.76.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]




