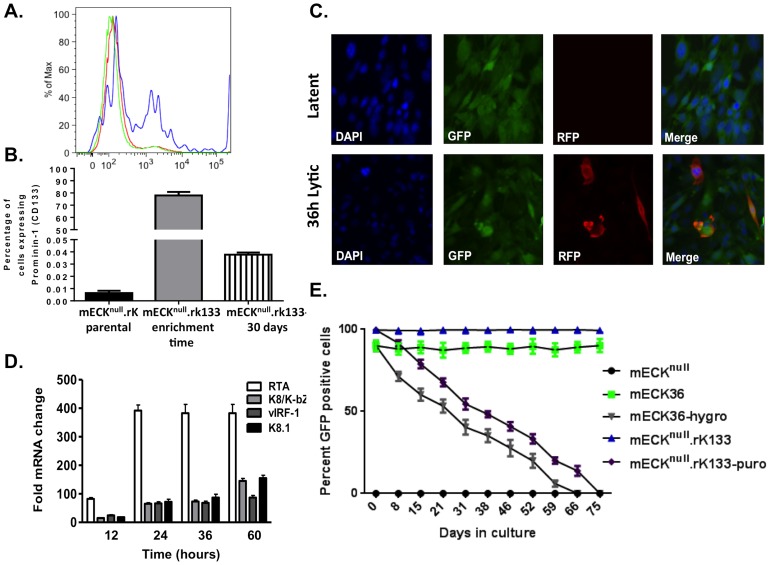
Figure 3. CD133/Prominin-1 enrichment of the mECKnull.rK results in a lytically-inducible population that harbors KSHV as an episome.
(A) mECKnull.rK were harvested from cell culture and CD133-expressing cells were positively selected using immunomagnetic beads. The histogram shows the parental population (green), the CD133 enriched (mECKnull.rK133) (blue) and the CD133-depleted populations (red) were analyzed by flow cytometry to confirm CD133 enrichment. A representative histogram is shown. (B) Graph depicting the percentage of CD133 expression in the mECKnull.rK, prior to CD133-enrichment, the percentage of CD133 in the enriched population of mECKnull.rK133 immediately post-enrichment, and the mECKnull.rK133 one month after CD133 enrichment. Error bars represent standard deviation between triplicate wells. (C) In vitro, mECKnull.rK133 express GFP, indicating rKSHV.219 infection, and maintain tight latency as determined by the absence of RFP expression (top panels). When treated with TSA lytic replication is induced as determined by the expression of RFP (bottom panels). (D) RFP induction after lytic reactivation is concurrent with the upregulation of KSHV lytic gene expression as determined by qRT-PCR for RTA, K8, vIRF-1 and K8.1. Error bars represent the SD of duplicate wells. Data are representative of three independent experiments. (E) mECKnull.rK133 cells are episomally-infected with KSHV. mECKnull.rK133 cells were serially passaged in the absence of hygromycin and GFP was measured by flow cytometry. While almost 100% of cells are GFP positive at day 0, by day 70, they are 100% GFP negative, suggesting that the virus exists as an episome in the murine cells.