Abstract
Purpose
Smoking is the primary etiologic risk factor for bladder cancer and has been implicated in mechanisms of chemoresistance. We investigated smoking as a potential predictor for pathologic outcomes after neoadjuvant chemotherapy (NC) and radical cystectomy (RC) for muscle-invasive bladder cancer.
Methods
We identified 139 patients treated with neoadjuvant cisplatin-based chemotherapy followed by RC for T2-4aN0M0 bladder cancer. Logistic regression was used to evaluate associations between smoking characteristics and pathologic outcomes (pT0, complete response; pT0/pTis/pT1, any response). In a secondary analysis, multivariate Cox regression was used to assess associations between smoking and recurrence-free and cancer-specific survival.
Results
Our cohort consisted of 99 (71 %) males, with a median age of 65 (interquartile range 56, 71). Prevalence of never, former, and current smokers was 25, 45, and 29 %, respectively. In total, 63 patients experienced disease recurrence, 39 died of disease, and 11 died of other causes. There were no statistically significant associations between smoking characteristics and complete (p = 0.5) or any (p = 0.2) pathologic response to NC. Similarly, we did not find any association between smoking characteristics and recurrence (p = 0.6) or cancer-specific survival (p = 0.9).
Conclusions
In this series, smoking characteristics were not found to be predictive of pathologic response after NC and RC, although this analysis was limited by the small study sample size. However, the harmful effects of smoking warrants continued emphasis on smoking cessation counseling in bladder cancer patients.
Keywords: Smoking, Neoadjuvant chemotherapy, Cisplatin, Radical cystectomy, Bladder cancer
Introduction
Approximately 20–40 % of bladder tumors are high-grade, muscle-invasive cancers at diagnosis [1]. In the last decade, randomized controlled trials have shown that administration of neoadjuvant cisplatin-based chemotherapy for muscle-invasive disease confers a survival advantage over cystectomy alone [2-4]. In several of these trials, patients with pathologic response to neoadjuvant chemotherapy (NC), as determined by absence of residual tumor or downstaging to non-muscle-invasive disease at the time of radical cystectomy (RC), experienced the best survival outcomes.
The most common etiologic risk factor for bladder cancer is smoking, accounting for up to 60 % of all cases [5]. Smoking intensity and duration have been shown to be linearly related to the increased risk of disease development [5-7]. However, smoking cessation is also associated with decreased risk of developing disease [5, 6]. Studies of bladder cancer prognosis also suggest a cancer-specific benefit to smoking cessation [8, 9]. Cell line and animal studies have shown that metabolites of tobacco smoke are involved in tumorigenesis and cell proliferation in bladder cancer and other solid organ malignancies [10, 11]. There is emerging molecular evidence that cigarette smoking is associated with response to standard chemotherapeutic agents, including cisplatin. Exposure of cancer cell lines to nicotine and other metabolites of cigarette smoke induces changes in cell proliferation and apoptotic pathways normally activated by cisplatin [12-16].
In this study, our aim was to identify an association between smoking characteristics and pathological response to NC. We studied a cohort of bladder cancer patients at our institution, with the hypothesis that smokers are at higher risk of chemoresistance and residual muscle-invasive disease at RC. In a secondary analysis, we also assessed for an association between smoking and the endpoints of recurrence and cancer-specific death in patients receiving NC.
Patients and methods
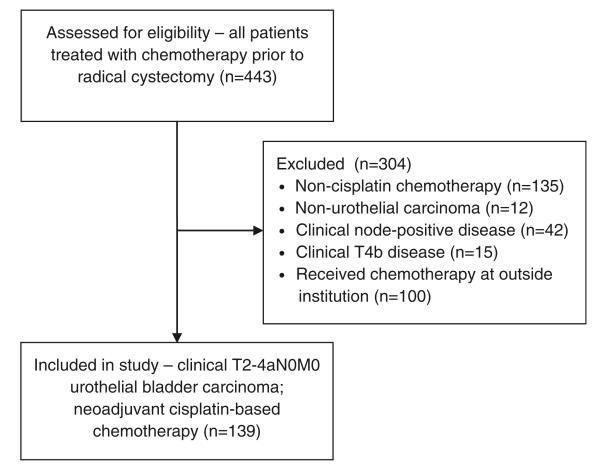
With Institutional Review Board approval, we retrospectively reviewed clinical records of 443 patients treated with systemic chemotherapy followed by RC and urinary diversion for primary bladder urothelial carcinoma at our institution between May 1990 and September 2011. From this cohort, we excluded patients who: received non-cisplatin-based NC; had a diagnosis of non-urothelial bladder cancer; received chemotherapy for clinical T4b or node-positive disease at diagnosis; or received chemotherapy at outside institutions prior to cystectomy. Our final cohort consisted of 139 patients with muscle-invasive urothelial bladder cancer (T2-T4aN0M0) who received neoadjuvant cisplatin-based chemotherapy and RC at our institution (Fig. 1). All patients were clinically staged by bimanual examination after endoscopy, in conjunction with appropriate imaging studies to detect evidence of extravesical extension, local nodal involvement, and distant metastases [17].
Fig. 1.
Flow diagram depicting initial number of patients assessed for eligibility, exclusion criteria, and final study cohort
Our primary outcome was pathologic response to cisplatin-based NC, determined by final pathologic stage at time of RC by the 2010 AJCC TNM staging system [17]. Patients were defined as having a complete response if there was no evidence of residual carcinoma (pT0) and partial response if there was residual, but non-muscle-invasive, disease (pTa/pTis/pT1). Patients were defined as having no response if there was residual muscle-invasive disease (pT2-4) or any evidence of lymph node involvement (pN?) or distant metastatic disease (pM?). All transurethral (TUR) and RC surgical specimens were reviewed by board-certified genitourinary pathologists at our institution according to a standard template. As an additional outcome measure, we also determined recurrence-free and cancer-specific survival. Disease recurrence was defined as pathologically confirmed carcinoma in the remaining urothelial tract, local operative field, or at distant metastatic sites. Patients were also considered to have disease recurrence if a radiologically suspicious metastasis was the basis for initiation of palliative or salvage systemic therapy. Cause of death was determined by chart review.
Smoking characteristics were obtained from patient-reported histories of tobacco use at time of initial consultation, which was routinely collected throughout the study period. Variables of interest included smoking status (active smokers, former smokers, and never smokers); quantity of cigarettes smoked per day (20 cigarettes per pack); duration of cigarette smoking (in years); and quit date for former smokers. Patients were considered active smokers if they reported smoking at diagnosis or if their quit date was within one year of diagnosis. This definition of active smoking has been used in prior studies of bladder cancer treatment outcomes and is based on the observation that many patients will have asymptomatic lesions prior to diagnosis [9, 18, 19]. Furthermore, bladder cancer patients who are actively smoking at time of diagnosis have been shown to have worse cancer-related outcomes [9, 18]. Duration of smoking and quantity of cigarettes smoked were used to calculate smoking pack-years.
Descriptive statistics were used to characterize patient and tumor characteristics, including smoking status, packs per day, and pack-years. Logistic regression was used to create a “base” model using the predictors of patient age, gender, clinical stage (cT2 vs. cT3/T4), and age-adjusted Charlson comorbidity score (categorized as 0–2, 3–4, and 5+). We individually incorporated each smoking characteristic into this model to evaluate its association with the pathologic outcomes of interest. Multivariate Cox regression was used to determine if smoking characteristics were associated with recurrence or cancer-specific death. A competing risk regression model was also used to evaluate associations of smoking characteristics with time to death. All statistical analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
Results
Patient characteristics are described in Table 1. The median age for our cohort was 65 [interquartile range (IQR) 56–71] and 99 (71 %) were male. The majority of patients had clinical T2 disease (60 %). The most common cisplatin-based NC regimen was gemcitabine and cisplatin alone in 102 (73 %). Other regimens included methotrexate–vinblastine–doxorubicin–cisplatin, ifosfamide–paclitaxel–cisplatin, cisplatin and etoposide, or one of these combinations with an additional investigational trial therapy. There were 35 never smokers, 63 former smokers, and 41 active smokers.
Table 1.
Patient characteristics
| n=139 | |
|---|---|
| Age, years, median (IQR) | 65 (56–71) |
| Gender, n (%) | |
| Male | 99 (71) |
| Female | 40 (29) |
| Body mass index, median (IQR) | 27.7 (24.9–30.6) |
| Age-adjusted Charlson score, n (%) | |
| 0 | 11 (8) |
| 1 | 28 (20) |
| 2 | 33 (24) |
| 3 | 38 (27) |
| 4 | 17 (12) |
| 5+ | 12 (9) |
| Clinical T stage, n (%) | |
| T2 | 84 (60) |
| T3 | 41 (29) |
| T4 | 14 (10) |
| Pathologic T stagea, n (%) | |
| pT0 | 24 (17) |
| pTis | 22 (16) |
| pT1 | 11 (8) |
| pT2 | 17 (12) |
| pT3 | 57 (41) |
| pT4 | 8 (6) |
| Pathologic N stage, n (%) | |
| pN0 | 99 (71) |
| pN+ | 37 (27) |
| pNx | 3 (2) |
| Smoking status, n (%) | |
| Never | 35 (25) |
| Former | 63 (45) |
| Activeb | 41 (29) |
| Packs per day, median (IQR) | 1.0 (0.0–1.5) |
| Pack-years, median (IQR) | 20.0 (0.0–45.0) |
Four patients <pT2 were also node-positive at cystectomy
Any reported smoking history within one year of initial diagnosis
In total, 53 patients (38 %) exhibited pathologic response (<pT2N0) at time of RC, 22 with complete response (pT0), and 31 with partial response (pTis or pT1). During follow-up, 63 patients experienced disease recurrence, 39 died of disease, and 11 died of other causes. The median follow-up for patients who did not recur or die was 46 and 40 months, respectively.
Table 2 shows the association between each smoking characteristic and each pathologic outcome. Smoking status was not significantly associated with either complete response (p = 0.5) or any response (p = 0.2), adjusting for age, gender, clinical stage, and age-adjusted Charlson score. For example, former smokers had 50 % decreased odds for complete response compared to never smokers, but this result was not significant, with a 95 % confidence interval (CI) ranging between 0.15 and 1.73. Therefore, being a former smoker could increase odds of complete response by 73 %, but could also decrease odds by 85 %. Smoking quantity, measured in packs per day, was associated with 35 % decreased odds for complete response, but this also was not statistically significant (95 % CI 0.35–1.20; p = 0.2). Results were similar for smoking volume: For every 10-year increase in pack-years smoked, there was an 8 % decrease in odds of complete response, but this was not statistically significant (95 % CI 0.77–1.09; p = 0.3). In each model, only clinical T stage was significantly associated with both complete (all p ≤ 0.011) and any pathologic response (all p ≤ 0.003). For example, compared to patients with cT2 disease, patients with cT3 or cT4 disease had significantly decreased odds for having complete response (OR 0.17, 95 % CI 0.04, 0.66; p = 0.006) or any pathologic response (OR 0.27, 95 % CI 0.12, 0.63; p = 0.003) in the model incorporating smoking status.
Table 2.
Logistic regression models of complete response and complete or any response (complete or partial response) after neoadjuvant chemotherapy and radical cystectomy adjusted for age, gender, clinical stage (T3/4 vs. T2), age-adjusted Charlson score (0–2, 3–4, 5+), and smoking characteristics included individually
| Complete responsea |
Complete or partial responseb |
|||
|---|---|---|---|---|
| OR (95 %CI) | p value | OR (95 % CI) | p value | |
| Smoking status | ||||
| Age (per 10 years) | 1.14 (0.58, 2.27) | 0.7 | 1.07 (0.64, 1.79) | 0.8 |
| Male | 0.75 (0.23, 2.47) | 0.6 | 1.20 (0.48, 3.01) | 0.7 |
| Clinical stage (T3/4 vs. T2) | 0.17 (0.04, 0.66) | 0.011 | 0.27 (0.12, 0.63) | 0.003 |
| Charlson score | 0.8 | 0.9 | ||
| 0–2 | Ref. | - | Ref. | - |
| 3–5 | 0.95 (0.25, 3.60) | - | 1.04 (0.37, 2.88) | - |
| 5+ | 2.31 (0.19, 28.62) | - | 0.53 (0.05, 5.74) | - |
| Smoking status | 0.5 | 0.2 | ||
| Never | Ref. | - | Ref. | - |
| Former | 0.50 (0.15, 1.73) | - | 0.88 (0.34, 2.27) | - |
| Active | 0.95 (0.29, 3.16) | - | ]2.04 (0.76, 5.51) | - |
| Packs per day | ||||
| Age (per 10 years) | 1.07 (0.55, 2.07) | 0.8 | 0.99 (0.60, 1.63) | 0.9 |
| Male | 0.70 (0.22, 2.22) | 0.5 | 1.11 (0.46, 2.68) | 0.8 |
| Clinical stage (T3/4 vs. T2) | 0.16 (0.04, 0.64) | 0.009 | 0.27 (0.12, 0.62) | 0.002 |
| Charlson score | 0.8 | 0.8 | ||
| 0–2 | Ref. | - | Ref. | - |
| 3–5 | 1.02 (0.27, 3.85) | - | 0.99 (0.37, 2.70) | - |
| 5+ | 2.38 (0.19, 29.93) | - | 0.50 (0.05, 5.44) | - |
| Packs per day | 0.65 (0.35, 1.20) | 0.2 | 0.88 (0.58, 1.31) | 0.5 |
| Pack-years | ||||
| Age (per 10 years) | 1.07 (0.55, 2.07) | 0.8 | 0.99 (0.60, 1.63) | 0.9 |
| Male | 0.64 (0.20, 2.01) | 0.4 | 1.08 (0.45, 2.60) | 0.9 |
| Clinical stage (T3/4 vs. T2) | 0.16 (0.04, 0.64) | 0.009 | 0.27 (0.12, 0.62) | 0.002 |
| Charlson score | 0.8 | 0.8 | ||
| 0–2 | Ref. | - | Ref. | - |
| 3–5 | 0.99 (0.26, 3.70) | - | 0.97 (0.36, 2.62) | - |
| 5+ | 2.35 (0.18, 30.07) | - | 0.47 (0.04, 5.23) | - |
| Pack-years (per 10 years) | 0.92 (0.77, 1.09) | 0.3 | 1.00 (0.89, 1.13) | 0.9 |
Complete response (pT0)
Partial response (pTis or pT1)
OR odds ratio, CI confidence interval
Results from our Cox models for recurrence and cancer-specific death are shown in Table 3. None of the smoking characteristics tested was significantly associated with recurrence or cancer-specific death, after adjusting for the same variables listed above. There were no significant differences in odds for recurrence (p = 0.6) or cancer-specific death (p = 0.9) amongst never, former, and current smokers. Smoking quantity and volume characteristics were associated with increased odds for recurrence and cancer-specific death, but were not statistically significant (all p > 0.1). As seen with pathologic response, only clinical T stage was significantly associated with recurrence (all p ≤ 0.006) and cancer-specific death (all p ≤ 0.001) in each model. In the model including smoking status, patients with higher clinical T stage (cT3 or cT4) were 2.15 times more likely to recur (95 % CI 1.25, 3.69; p = 0.001) and 3.41 times more likely to experience cancer-specific death (95 % CI 1.68, 6.90; p = 0.001) that those with cT2 disease. Since smoking is associated with an increased risk of death from other smoking-related health problems, we used a competing risk regression to evaluate the association between smoking and cancer-specific death. Similar to our Cox model, however, competing risk regression did not reveal any statistically significant associations.
Table 3.
Cox regression models of recurrence and cancer-specific death after neoadjuvant chemotherapy and radical cystectomy, adjusted for age, gender, clinical stage (T3/4 vs. T2), age-adjusted Charlson score (0–2, 3–4, 5+), and smoking characteristics included individually
| Recurrence |
Cancer-specific death |
|||
|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | |
| Smoking status | ||||
| Age (per 10 years) | 1.04 (0.74, 1.47) | 0.8 | 0.91 (0.60, 1.39) | 0.7 |
| Male | 1.37 (0.74, 2.51) | 0.3 | 2.15 (0.96, 4.85) | 0.064 |
| Clinical stage (T3/4 vs. T2) | 2.15 (1.25, 3.69) | 0.006 | 3.41 (1.68, 6.90) | 0.001 |
| Charlson score | 0.6 | 0.8 | ||
| 0–2 | Ref. | - | Ref. | - |
| 3–5 | 0.73 (0.36, 1.48) | - | 0.93 (0.37, 2.29) | - |
| 5+ | 0.55 (0.12, 2.56) | - | 0.47 (0.06, 4.02) | - |
| Smoking status | 0.6 | 0.9 | ||
| Never | Ref. | - | Ref. | - |
| Former | 1.24 (0.66, 2.31) | - | 0.90 (0.40, 2.03) | - |
| Active | 0.91 (0.44, 1.84) | - | 1.07 (0.44, 2.60) | - |
| Packs per day | ||||
| Age (per 10 years) | 1.08 (0.76, 1.54) | 0.7 | 0.91 (0.60, 1.38) | 0.7 |
| Male | 1.40 (0.76, 2.59) | 0.3 | 2.00 (0.90, 4.46) | 0.089 |
| Clinical stage (T3/4 vs. T2) | 2.17 (1.25, 3.76) | 0.006 | 3.21 (1.59, 6.49) | 0.001 |
| Charlson score | 0.5 | 0.8 | ||
| 0–2 | Ref. | - | Ref. | - |
| 3–5 | 0.69 (0.34, 1.41) | - | 0.86 (0.35, 2.09) | - |
| 5+ | 0.54 (0.12, 2.52) | - | 0.49 (0.06, 4.15) | - |
| Packs per day | 1.25 (0.95, 1.63) | 0.11 | 1.17 (0.81, 1.70) | 0.4 |
| Pack-years | ||||
| Age (per 10 years) | 1.07 (0.75, 1.52) | 0.7 | 0.90 (0.59, 1.37) | 0.6 |
| Male | 1.44 (0.78, 2.67) | 0.2 | 2.05 (0.92, 4.58) | 0.081 |
| Clinical stage (T3/4 vs. T2) | 2.18 (1.26, 3.78) | 0.006 | 3.25 (1.60, 6.60) | 0.001 |
| Charlson score | 0.5 | 0.8 | ||
| 0–2 | Ref. | - | Ref. | - |
| 3–5 | 0.71 (0.35, 1.44) | - | 0.86 (0.36, 2.10) | - |
| 5+ | 0.52 (0.11, 2.43) | - | 0.46 (0.06, 3.90) | - |
| Pack-years (per 10 years) | 1.05 (0.97, 1.14) | 0.2 | 1.05 (0.94, 1.17) | 0.4 |
HR hazard ratio, CI confidence interval
Discussion
The addition of NC confers a survival advantage to patients with muscle-invasive bladder cancer undergoing cystectomy, but only ~40 % demonstrate pathologic evidence of treatment response [2, 3, 20]. Several groups have reported that pathologic responders have improved overall survival over those with residual muscle-invasive disease, with the best outcomes experienced by those with complete response (pT0) [20, 21]. Therefore, the identification of factors that may predict sensitivity or resistance to NC remains an important area of clinical and translational research.
We hypothesized that cigarette smoking—a potentially modifiable patient behavior—may predict pathologic response in these patients. As expected, the majority of patients (75 %) in our cohort reported a history of smoking and nearly a third reported smoking within a year of diagnosis. We observed a 38 % pathologic response rate to NC, consistent with other studies [20]. However, we did not find any significant associations between smoking status, volume, or duration and pathologic outcomes after NC and RC. Likewise, we did not find any associations between smoking characteristics and recurrence-free and cancer-specific survival, even after addressing competing risks associated with tobacco exposure.
Differences in underlying tumor biology determine chemosensitivity in bladder cancer and can be affected by environmental carcinogens. Cigarette smoking is the primary risk factor for bladder urothelial carcinoma. Furthermore, smoking duration and intensity have a linear relationship with risk of bladder cancer diagnosis [5-7]. Smoking cessation, on the other hand, lowers risk of bladder cancer, even in formerly heavy smokers [8, 9]. Tobacco-related tumorigenesis is a multifaceted process involving multiple carcinogenic tobacco metabolites such as nicotine-derived nitrosamine ketones and polyaromatic hydrocarbons. These components promote tumor formation through direct DNA damage, mutating oncogenes and tumor suppressors, or by modifying key signal transduction pathways [10, 11]. Smoking has also been implicated in mechanisms of resistance to cisplatin chemotherapy in bladder cancer and other solid organ malignancies [12, 13, 15, 16]. For example, STAT3, a transcription factor involved in cell growth and apoptosis, is activated by nicotine and leads to chemoresistance, while inhibition of STAT3 restores chemosensitivity in vitro [12, 13]. Nicotine activates other important cell signaling pathways involved in cell survival and inhibition of cisplatin-induced apoptosis. Akt, a serine–threonine phosphatase in the PI3K/Akt/mTOR signaling pathway, is activated by exposure to nicotine in several cancer cell lines [14, 15]. Importantly, in oral cancer cell lines exposed to nicotine, treatment with the PI3K pathway inhibitor LY294002 restored cisplatin-mediated apoptosis [15]. These studies highlight the potential benefit of molecular therapies directed against these cell survival pathways—in addition to cigarette cessation—in patients resistant to chemotherapy. The emergence of high-throughput genomic profiling may also identify novel prognostic markers for cisplatin sensitivity in cancer patients [22, 23]. Prospective identification of patients likely to respond or to fail conventional chemotherapy may enhance overall treatment outcomes for patients with bladder cancer.
Our results should be interpreted in the context of the study design and its limitations, including its retrospective nature and small sample size. Smoking characteristics were obtained by chart review of patient-reported histories taken at the time of initial consultation. Although our review was comprehensive, patient self-report of smoking duration and volume is subject to recall bias, and quit dates may not be faithfully reported. Future studies using alternative, objective measures of smoking exposure, such as serum cotinine levels, may better assess smoking status at initiation of treatment [24]. Our sample size was small and limited to patients treated at a single, tertiary referral center. This was reflected in the wide confidence intervals seen in several of our analyses. However, some of our results indicate that a larger cohort of similar patients may confirm some of the observed associations. For example, smoking status (packs per day) was associated with a 25 % increase in odds for recurrence and a 17 % increase in odds for cancer-specific death. Although not statistically significant (p = 0.1), the 95 % CI for recurrence, for instance, ranged from 0.95 to 1.63. This suggests that smoking quantity, in effect, might actually reduce a patient’s odds of recurrence by 5 %, but might increase odds to a greater degree, 63 %. Thus, smoking reduction may have potential benefits that warrant further study in patients receiving NC and cystectomy for bladder cancer.
Other factors may have influenced our results but were not captured in the data set. Patients who were not able to receive cisplatin at time of diagnosis, due to renal insufficiency or preexisting hearing loss, were not included. Furthermore, we did not include patients who never proceeded to RC due to evidence of disease progression on chemotherapy. We also did not capture post-diagnosis smoking characteristics, including potential smoking reduction or cessation during chemotherapy and before RC. Prior studies in non-muscle-invasive bladder cancer have shown poorer cancer-related outcomes in patients who continued to smoke after diagnosis [8, 18, 25]. In muscle-invasive disease, a recent multicenter retrospective study reported that current smokers, compared to former smokers, were at increased risk of disease recurrence after RC. Cumulative smoking exposure was also associated with disease recurrence and cancer-specific mortality, with the worst outcomes experienced by heavy long-term smokers (>20 cigarettes per day for >20 years) [9]. Although patients receiving NC were not included in this analysis, smoking status appears to influence cancer-specific outcomes after diagnosis and initiation of appropriate therapy. Given the potential for chemoresistance in active smokers, future studies should consider prospective collection and biochemical verification of smoking status in patients receiving chemotherapy and cystectomy for muscle-invasive disease [26].
Smoking was not associated with pathologic outcome or survival in our study. However, promotion of smoking cessation should continue to be an integral component of multimodal bladder cancer care, due to the numerous deleterious effects of smoking on overall health beyond its effects on cancer. Patients should be therefore be counseled regarding these risks of smoking and directed to effective and appropriate cessation therapies. Unfortunately, smoking cessation continues to be an underutilized intervention for bladder cancer patients [27].
Conclusion
Despite emerging evidence for the role of smoking in chemoresistance, we did not find any significant associations of smoking with pathologic and cancer-specific outcomes after NC and RC, although our single-institution series was limited by small sample size. Future prospective studies investigating the role of smoking and treatment response in bladder cancer would be well served to obtain biochemical verification of self-reported smoking status. Smoking cessation counseling should be an integral part of bladder cancer care given the known detrimental effect smoking has on cancer-specific and overall health outcomes.
Acknowledgments
This study was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers. Dr. Sfakianos is a research fellow in urologic oncology supported by the NIH T32-CA82088 training grant.
References
- 1.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP, Jr, Raghavan D, Crawford ED. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148. doi:10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–2177. doi: 10.1200/JCO.2010.32.3139. doi:10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advanced Bladder Cancer Meta-analysis C Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–205. doi: 10.1016/j.eururo.2005.04.006. discussion 205-206. doi:10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Boffetta P. Tobacco smoking and risk of bladder cancer. Scand J Urol Nephrol Suppl. 2008;218:45–54. doi: 10.1080/03008880802283664. doi:10.1080/03008880802283664. [DOI] [PubMed] [Google Scholar]
- 6.Lerner SP, Grossman HB, Messing EM, Kibel AS, Stephenson A, Gee JR, O’Donnell MA, Reid RD, Kamat AM, Parnes HL, House MG. BCAN think tank session 3: prevention of bladder cancer. Urol Oncol. 2010;28(3):338–342. doi: 10.1016/j.urolonc.2009.06.018. doi:10.1016/j.urolonc.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Zeegers MP, Goldbohm RA, van den Brandt PA. A prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands) Cancer Causes Control. 2002;13(1):83–90. doi: 10.1023/a:1013954932343. doi:10.1023/A:1013954932343. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Shun CT, Huang KH, Huang CY, Tsai YC, Yu HJ, Pu YS. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int. 2007;100(2):281–286. doi: 10.1111/j.1464-410X.2007.06873.x. discussion 286. doi:10.1111/j.1464-410X.2007.06873.x. [DOI] [PubMed] [Google Scholar]
- 9.Rink M, Zabor EC, Furberg H, Xylinas E, Ehdaie B, Novara G, Babjuk M, Pycha A, Lotan Y, Trinh QD, Chun FK, Lee RK, Karakiewicz PI, Fisch M, Robinson BD, Scherr DS, Shariat SF. Impact of smoking and smoking cessation on outcomes in bladder cancer patients treated with radical cystectomy. Eur Urol. 2012 doi: 10.1016/j.eururo.2012.11.039. doi:10.1016/j.eururo.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Mousa S, Mousa SA. Cellular and molecular mechanisms of nicotine’s pro-angiogenesis activity and its potential impact on cancer. J Cell Biochem. 2006;97(6):1370–1378. doi: 10.1002/jcb.20741. doi:10.1002/jcb.20741. [DOI] [PubMed] [Google Scholar]
- 11.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J (Off Publ Fed Am Soc Exp Biol) 2006;20(12):2093–2101. doi: 10.1096/fj.06-6191com. doi:10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- 12.Chen RJ, Ho YS, Guo HR, Wang YJ. Long-term nicotine exposure-induced chemoresistance is mediated by activation of Stat3 and downregulation of ERK1/2 via nAChR and beta-adrenoceptors in human bladder cancer cells. Toxicol Sci. 2010;115(1):118–130. doi: 10.1093/toxsci/kfq028. doi:10.1093/toxsci/kfq028. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Kim D, Gao J, Kurtyka C, Chen H, Yu C, Wu D, Mittal A, Beg AA, Chellappan SP, Haura EB, Cheng JQ. IKBKE is induced by STAT3 and tobacco carcinogen and determines chemosensitivity in non-small cell lung cancer. Oncogene. 2013;32(2):151–159. doi: 10.1038/onc.2012.39. doi:10.1038/onc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Tsurutani J, Castillo SS, Brognard J, Granville CA, Zhang C, Gills JJ, Sayyah J, Dennis PA. Tobacco components stimulate Akt-dependent proliferation and NFkappaB-dependent survival in lung cancer cells. Carcinogenesis. 2005;26(7):1182–1195. doi: 10.1093/carcin/bgi072. doi:10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Huang H, Pan C, Zhang B, Liu X, Zhang L. Nicotine inhibits apoptosis induced by cisplatin in human oral cancer cells. Int J Oral Maxillofac Surg. 2007;36(8):739–744. doi: 10.1016/j.ijom.2007.05.016. doi:10.1016/j.ijom.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Chang X, Ravi R, Pham V, Bedi A, Chatterjee A, Sidransky D. Adenylate kinase 3 sensitizes cells to cigarette smoke condensate vapor induced cisplatin resistance. PLoS ONE. 2011;6(6):e20806. doi: 10.1371/journal.pone.0020806. doi:10.1371/journal.pone.0020806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SB. AJCC cancer staging manual. 7th Springer; New York: 2010. [Google Scholar]
- 18.Fleshner N, Garland J, Moadel A, Herr H, Ostroff J, Trambert R, O’Sullivan M, Russo P. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer. 1999;86(11):2337–2345. [PubMed] [Google Scholar]
- 19.Ehdaie B, Furberg H, Zabor EC, Ostroff JS, Shariat SF, Bochner BH, Coleman JA, Dalbagni G. Impact of smoking status at diagnosis on disease recurrence and death in upper tract urothelial carcinoma. BJU Int. 2012 doi: 10.1111/j.1464-410X.2012.11260.x. doi:10.1111/j.1464-410X.2012.11260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonpavde G, Goldman BH, Speights VO, Lerner SP, Wood DP, Vogelzang NJ, Trump DL, Natale RB, Grossman HB, Crawford ED. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115(18):4104–4109. doi: 10.1002/cncr.24466. doi:10.1002/cncr.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassouf W, Spiess PE, Brown GA, Munsell MF, Grossman HB, Siefker-Radtke A, Dinney CP, Kamat AM. P0 stage at radical cystectomy for bladder cancer is associated with improved outcome independent of traditional clinical risk factors. Eur Urol. 2007;52(3):769–774. doi: 10.1016/j.eururo.2007.03.086. doi:10.1016/j.eururo.2007.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang RS, Johnatty SE, Gamazon ER, Im HK, Ziliak D, Duan S, Zhang W, Kistner EO, Chen P, Beesley J, Mi S, O’Donnell PH, Fraiman YS, Das S, Cox NJ, Lu Y, Macgregor S, Goode EL, Vierkant RA, Fridley BL, Hogdall E, Kjaer SK, Jensen A, Moysich KB, Grasela M, Odunsi K, Brown R, Paul J, Lambrechts D, Despierre E, Vergote I, Gross J, Karlan BY, Defazio A, Chenevix-Trench G, Dolan ME. Platinum sensitivity-related germline polymorphism discovered via a cell-based approach and analysis of its association with outcome in ovarian cancer patients. Clin Cancer Res (Off J Am Assoc Cancer Res) 2011;17(16):5490–5500. doi: 10.1158/1078-0432.CCR-11-0724. doi:10.1158/1078-0432.CCR-11-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friboulet L, Barrios-Gonzales D, Commo F, Olaussen KA, Vagner S, Adam J, Goubar A, Dorvault N, Lazar V, Job B, Besse B, Validire P, Girard P, Lacroix L, Hasmats J, Dufour F, Andre F, Soria JC. Molecular characteristics of ERCC1-negative versus ERCC1-positive tumors in resected NSCLC. Clin Cancer Res (Off J Am Assoc Cancer Res) 2011;17(17):5562–5572. doi: 10.1158/1078-0432.CCR-11-0790. doi:10.1158/1078-0432.CCR-11-0790. [DOI] [PubMed] [Google Scholar]
- 24.Benowitz NL, Schultz KE, Haller CA, Wu AH, Dains KM, Jacob P., 3rd Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol. 2009;170(7):885–891. doi: 10.1093/aje/kwp215. doi:10.1093/aje/kwp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lammers RJ, Witjes WP, Hendricksen K, Caris CT, Janzing-Pastors MH, Witjes JA. Smoking status is a risk factor for recurrence after transurethral resection of non-muscle-invasive bladder cancer. Eur Urol. 2011;60(4):713–720. doi: 10.1016/j.eururo.2011.07.010. doi:10.1016/j.eururo.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. doi:10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 27.Bjurlin MA, Goble SM, Hollowell CM. Smoking cessation assistance for patients with bladder cancer: a national survey of American urologists. J Urol. 2010;184(5):1901–1906. doi: 10.1016/j.juro.2010.06.140. doi:10.1016/j.juro.2010.06.140. [DOI] [PubMed] [Google Scholar]