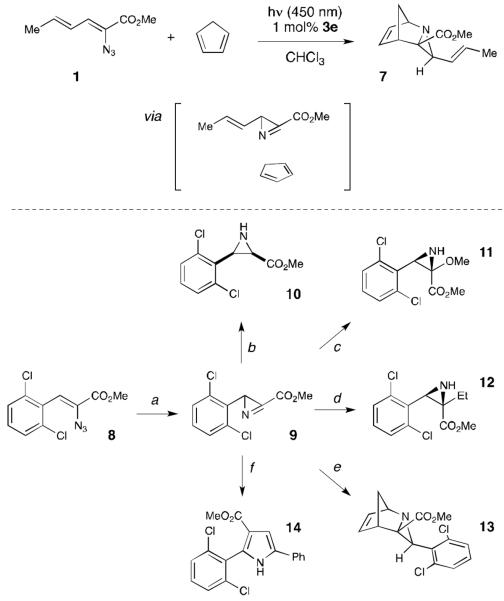
Scheme 2.
Manipulation of the azirine intermediate. (a) [Ir(dF(CF3)ppy)2(dtbbpy)](PF6) (1 mol %), CHCl3 (0.1 M), blue LEDs, rt, 8 h, 90% (b) Bu4NBH4, CH2Cl2, −78 °C, 15 min, 60% (c) NaOMe, MeOH, rt, 1 h, 93% (d) BEt3, EtI, CH2Cl2/hexanes, −40 °C, 30 min, 71% (e) Cp (5 equiv.), THF, rt, 1 h, 94% (f) 1-phenyl-2-(triphenylphosphanylidene)-ethanone (1 equiv.), CH2Cl2, rt, 24 h, 58%.