Table 1.
Initial investigations on triplet sensitization of dienyl azide 1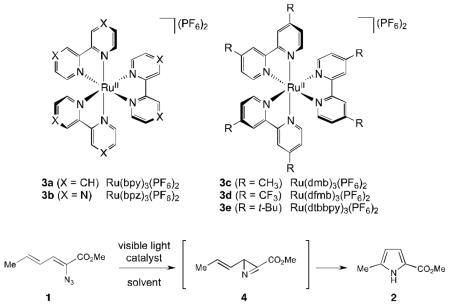
| entrya | catalyst (loading) | solvent (concentration) | time | yield 2 (yield 4)b |
|---|---|---|---|---|
| 1 | 3a (2.5 mol%) | MeCN (0.4 M) | 1.5 h | 12% (31%) |
| 2 | 3b (2.5 mol%) | MeCN (0.4 M) | 1.5 h | 9% (22%) |
| 3 | 3c (2.5 mol%) | MeCN (0.4 M) | 1.5 h | 21% (48%) |
| 4 | 3d (2.5 mol%) | MeCN (0.4 M) | 1.5 h | 17% (28%) |
| 5 | 3e (2.5 mol%) | MeCN (0.4 M) | 1.5 h | 25% (39%) |
| 6 | 3e (2.5 mol%) | DMSO (0.4 M) | 1.5 h | 22% (48%) |
| 7 | 3e (2.5 mol%) | acetone (0.4 M) | 1.5 h | 21% (41%) |
| 8 | 3e (2.5 mol%) | CH2CI2 (0.4M) | 1.5 h | 28% (32%) |
| 9 | 3e (2.5 mol%) | CHCI3 (0.4M) | 1.5 h | 30% (27%) |
| 10 | 3e (2.5 mol%) | CHCI3 (0.1 M) | 1.5 h | 34% (24%) |
| 11 | 3e (2.5 mol%) | CHCI3 (0.1 M) | 5 h | 94% (0%) |
| 12 | 3e (1 mol%) | CHCI3 (0.1 M) | 5 h | 91% (0%) |
| 13 | none | CHCI3 (0.1 M) | 5 h | 0% (0%) |
| 14c | 3e (1 mol%) | CHCI3 (0.1 M) | 5 h | 0% (0%) |
| 15d | 3e (1 mol%) | CHCI3 (0.1 M) | 5 h | 73% (12%) |
| 16e | none | MeCN (0.1 M) | 5 h | 49% (0%) |
Reactions conducted using 0.25 mmol of 1 under air-free conditions with irradiation from a blue LED strip unless otherwise noted.
Yields determined by 1H NMR analysis using an internal standard.
Reaction conducted in the dark.
Reaction irradiated with a 23 W compact fluorescent lightbulb.
Reaction irradiated at 254 nm in a Rayonet reactor.
