Abstract
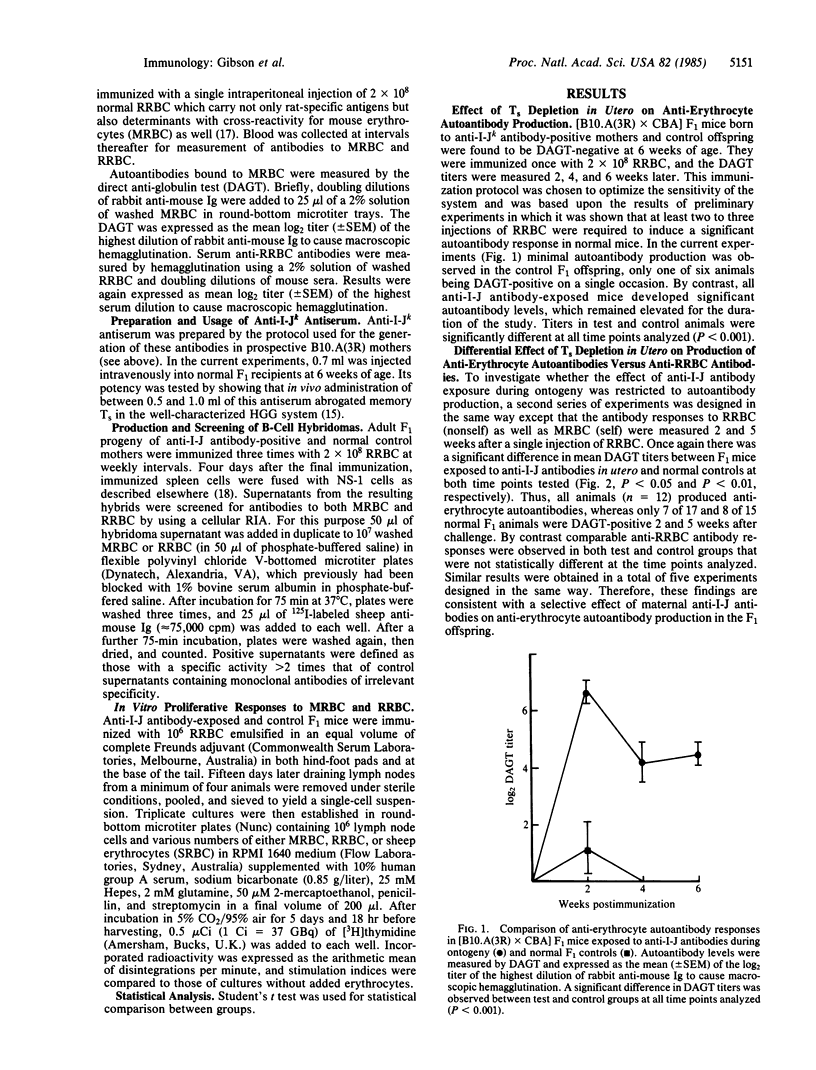
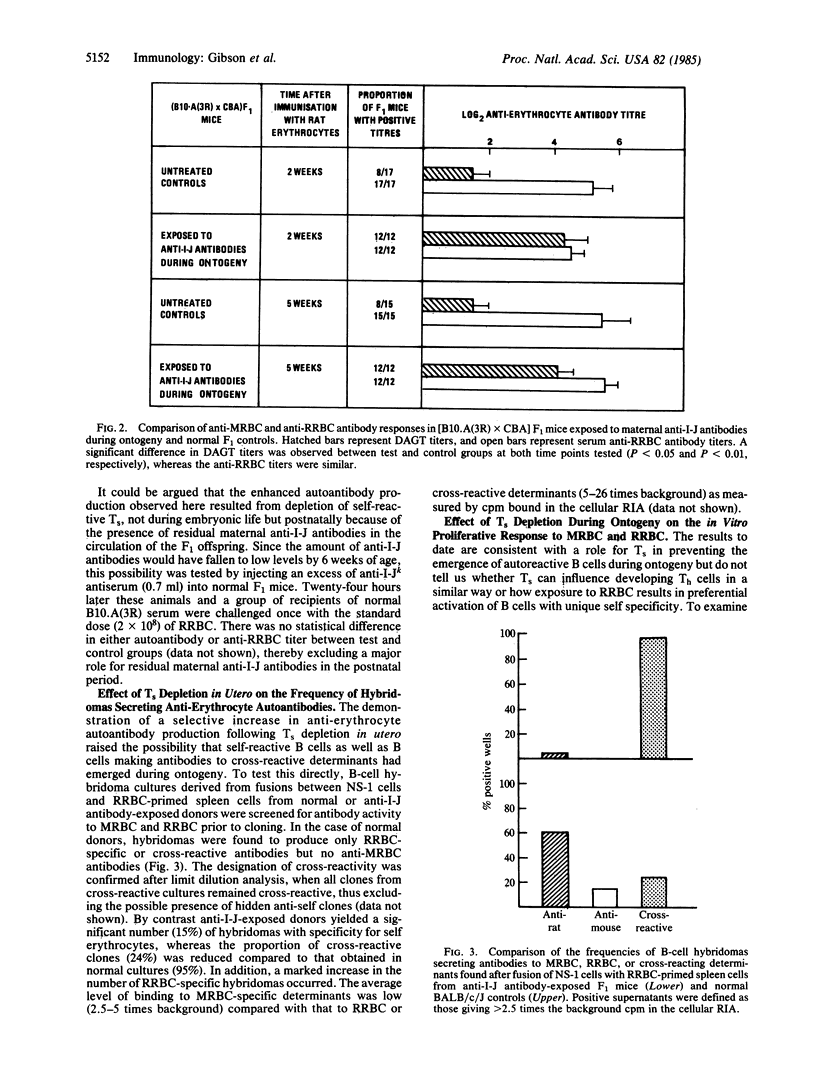
The potential role of suppressor T cells (Ts) in the induction of self-tolerance was investigated by eliminating I-J+ cells during ontogeny (I-J antigens are encoded by the I-J subregion of the murine major histocompatibility complex). To achieve this, F1 mice were exposed to anti-I-J antibodies via the transplacental route by mating B10.A(3R) females, preimmunized with B10.A(5R) cells, with CBA males. At 6 weeks of age, the offspring were injected with rat erythrocytes (RRBC) to induce erythrocyte autoantibodies. By comparison with age-matched controls, Ts-depleted mice produced significantly higher titers of autoantibody, whereas there was no difference in the antibody response of the two groups to the foreign determinants on the RRBC. The selective increase in autoantibody production was mirrored at the clonal level by the appearance of self-reactive B-cell hybridomas after fusion of RRBC-immune spleen cells with the NS-1 cell line. On the other hand, when helper cell function of RRBC-primed cells was measured in a T-cell proliferative assay, Ts depletion in utero resulted in enhanced T-cell activity to nonself (RRBC) but not to self (mouse erythrocyte) determinants. Thus, helper T cells recognizing nonself determinants on RRBC appeared to be responsible for activating self-specific B cells, presumably through linked recognition of different epitopes on mouse erythrocytes. Taken together, these findings indicate that elimination of I-J+ cells during ontogeny can lead to the appearance and activation of "forbidden" B-cell clones and points to a central role for Ts in induction as well as maintenance of self-tolerance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adda D. H., Beraud E., Depieds R. Evidence for suppressor cells in Lewis rats' experimental allergic encephalomyelitis. Eur J Immunol. 1977 Sep;7(9):620–623. doi: 10.1002/eji.1830070908. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Bogen S., Mozes E., Fuchs S. Induction of acetylcholine receptor-specific suppression. An in vitro model of antigen-specific immunosuppression in myasthenia gravis. J Exp Med. 1984 Jan 1;159(1):292–304. doi: 10.1084/jem.159.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boux H. A., Raison R. L., Walker K. Z., Musgrove E., Basten A. The surface expression of a tumor-associated antigen on human kappa myeloma cells. Eur J Immunol. 1984 Mar;14(3):216–222. doi: 10.1002/eji.1830140304. [DOI] [PubMed] [Google Scholar]
- Cooke A., Hutchings P. R., Playfair J. H. Suppressor T cells in experimental autoimmune haemolytic anaemia. Nature. 1978 May 11;273(5658):154–155. doi: 10.1038/273154a0. [DOI] [PubMed] [Google Scholar]
- Cox K. O., Howles A. Induction and regulation of autoimmune hemolytic anemia in mice. Immunol Rev. 1981;55:31–53. doi: 10.1111/j.1600-065x.1981.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Debré P. Stimulation of specific suppressor T cells in newborn responder mice by the terpolymer L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). Eur J Immunol. 1978 Sep;8(9):615–620. doi: 10.1002/eji.1830080902. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth B., Basten A., Loblay R. Induction of memory and effector suppressor T cells by perinatal exposure to antigen. Eur J Immunol. 1984 Mar;14(3):228–235. doi: 10.1002/eji.1830140306. [DOI] [PubMed] [Google Scholar]
- Gatenby P. A., Kotzin B. L., Engleman E. G. Induction of immunoglobulin secreting cells in the human autologous mixed leukocyte reaction: regulation by helper and suppressor lymphocyte subsets defined with monoclonal antibodies. J Immunol. 1981 Nov;127(5):2130–2135. [PubMed] [Google Scholar]
- Glazier A., Tutschka P. J., Farmer E. R., Santos G. W. Graft-versus-host disease in cyclosporin A-treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med. 1983 Jul 1;158(1):1–8. doi: 10.1084/jem.158.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. E., Cairns L., Rosen F. S., Borel Y. A natural model of immunologic tolerance. Tolerance to murine C5 is mediated by T cells, and antigen is required to maintain unresponsiveness. J Exp Med. 1982 Aug 1;156(2):567–584. doi: 10.1084/jem.156.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyczek K. K., Cantor H., Hayes C. E. T cell surface I-J glycoprotein. Concerted action of chromosome-4 and -17 genes forms an epitope dependent on alpha-D-mannosyl residues. J Exp Med. 1984 Jun 1;159(6):1604–1617. doi: 10.1084/jem.159.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. M., Okayasu I., Giraldo A. A., Beisel K. W., Sundick R. S., Rose N. R., David C. S., Audibert F., Chedid L. Tolerance to thyroglobulin by activating suppressor mechanisms. Ann N Y Acad Sci. 1982;392:191–209. doi: 10.1111/j.1749-6632.1982.tb36108.x. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Steinmetz M., Kobori J., Kraig E., Kapp J. A., Pierce C. W., Sorensen C. M., Suzuki G., Tada T., Hood L. RNA transcripts for I-J polypeptides are apparently not encoded between the I-A and I-E subregions of the murine major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5704–5708. doi: 10.1073/pnas.80.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIND P. E., BURNET F. M. Recombination between virulent and non-virulent strains of influenza virus. II. The behaviour of virulence markers on recombination. Aust J Exp Biol Med Sci. 1957 Feb;35(1):67–78. doi: 10.1038/icb.1957.8. [DOI] [PubMed] [Google Scholar]
- Loblay R. H., Fazekas de St Groth B., Pritchard-Briscoe H., Basten A. Suppressor T cell memory. II. The role of memory suppressor T cells in tolerance to human gamma globulin. J Exp Med. 1983 Mar 1;157(3):957–973. doi: 10.1084/jem.157.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukic M. L., Mitchison N. A. Self- and allo-specific suppressor T cells evoked by intravenous injection of F protein. Eur J Immunol. 1984 Aug;14(8):766–768. doi: 10.1002/eji.1830140820. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Herzenberg L. A., Okumura K., Herzenberg L. A., McDevitt H. O. A new I subregion (I-J) marked by a locus (Ia-4) controlling surface determinants on suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):699–712. doi: 10.1084/jem.144.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myburgh J. A., Mitchison N. A. Suppressor mechanisms in neonatally acquired tolerance to a Gross virus-induced lymphoma in rats. Transplantation. 1976 Sep;22(3):236–244. doi: 10.1097/00007890-197609000-00004. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Pierres M., Germain R. N., Dorf M. E., Benacerraf B. In vivo effects of anti-Ia alloantisera. I. Elimination of specific suppression by in vivo administration of antisera specific for I-J controlled determinants. J Exp Med. 1978 Mar 1;147(3):656–666. doi: 10.1084/jem.147.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W. Nature of the T-cell receptor. Both the T-cell receptor and antigen-specific T-cell-derived factors are coded for by V genes but express anti-self idiotypes indirectly determined by major histocompatibility complex genes. Scand J Immunol. 1979;10(5):387–393. doi: 10.1111/j.1365-3083.1979.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Analysis of autoimmunity through experimental models of thyroiditis and allergic encephalomyelitis. Adv Immunol. 1980;30:159–273. doi: 10.1016/s0065-2776(08)60196-0. [DOI] [PubMed] [Google Scholar]


