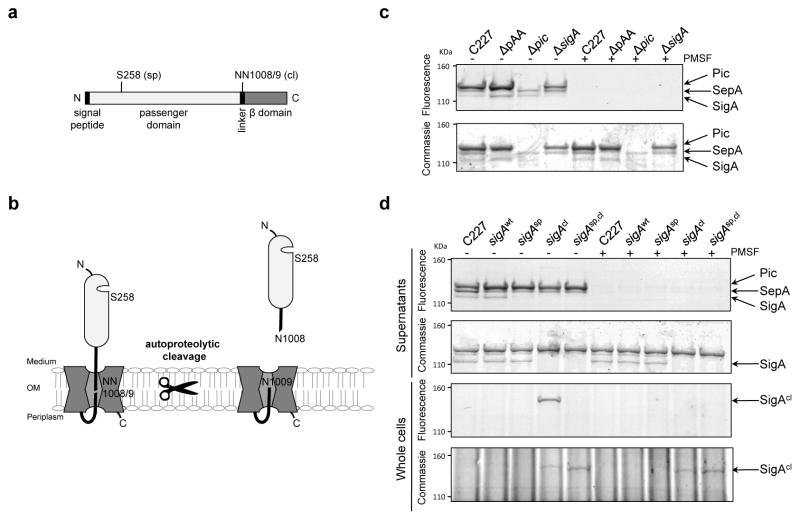
Figure 5. Activity of SPATEs from C227 and isogenic mutants.
(a) Domain structure and predicted catalytic serine (sp) and autocleavage sites (cl) in SigA. (b) Schematic of SigA topology and processing. (c) Activity-based protein profiling of secreted SPATEs from C227. Supernatant from overnight cultures of the indicated strains were reacted with the serine hydrolase probe (TAMRA-FP), run on SDS-PAGE gels, and scanned to detect fluorescence or stained with commassie to visualize total protein. Control samples were incubated with PMSF, which specifically abolishes serine protease activity, prior to addition of TAMRA-FP. (d) Activity-based protein profiling of C227, its ΔsigA derivative complemented with sigA-wt, or the indicated sigA point mutants. Whole cells or supernatants from overnight cultures were assayed as in (c). Full fluorescence-scanned blots and coomassie-stained gels are shown in Supplementary Fig. 8.