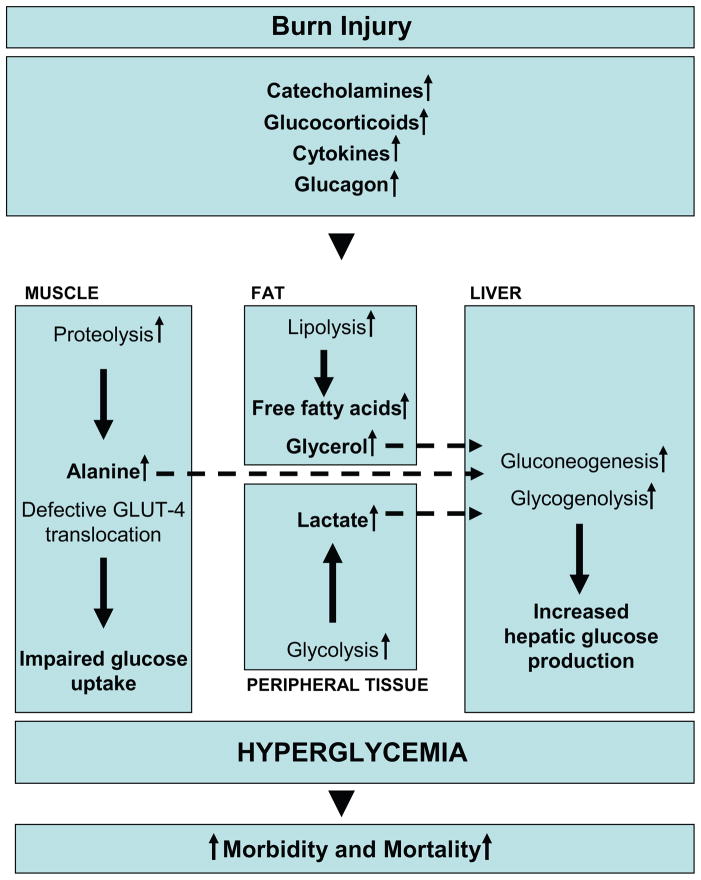
Figure 1. Metabolic changes underlying insulin resistance post-burn.
Marked and sustained increases in catecholamine, glucocorticoid, glucagon and cytokine secretion are thought to initiate the cascade of events leading to the acute hypermetabolic response to severe burn injury and oppose the anabolic effects of insulin. By enhancing adipose tissue lipolysis and skeletal muscle proteolysis, they increase gluconeogenic substrates, including glycerol, alanine and lactate, thus augmenting hepatic glucose production in burned patients. Catecholamine-mediated augmentation of hepatic glycogenolysis, as well as direct sympathetic stimulation of glycogen breakdown, further aggravates the hyperglycemia in response to stress. Catecholamines and cytokines, such as IL-1, IL-6, MCP-1 and TNF, have also been shown to impair glucose disposal via alterations of the insulin signaling pathway and GLUT-4 translocation, resulting in peripheral insulin resistance.