Abstract
Autografting of burn wounds results in generation of donor site wounds. Here we measured donor site wound protein Fractional Synthesis Rate (FSR) in a burn pediatric population and showed that FSR increases over time postsurgery and correlates with the length of hospital stay (LOS) normalized for total body surface area (TBSA) burn size. 3.9±1.1 days after the grafting surgery patients participated in a metabolic study consisting of continuous infusion of L-[ring-2H5]-phenylalanine and donor site wound punch biopsies. Donor site wound protein FSR was 10.4±7.5 %/day. Wound FSR demonstrated linear correlation with the time postsurgery (p < 0.05). Multiple regression analysis showed that LOS/TBSA correlated with donor site wound protein FSR and time postsurgery (p < 0.001) and the following equation describes the relationship: Estimated LOS/TBSA = (FSR - 12.95 – 1.414 × Postsurgery day)/(−17.8). This equation predicted that FSR corrected for the postsurgery day when the metabolic study was conducted accounted for 67 % of the variability (r2 = 0.673) in the LOS/TBSA. Donor site wound protein FSR correlated to LOS/TBSA of burn patients admitted to the intensive care unit. Measurement of protein deposition in regenerating donor site wound using stable isotope technique provides a quantitative measure of wound healing.
Keywords: Burn, donor site wound, protein synthesis, stable isotopes, length of hospitalization
INTRODUCTION
Early closure of the burn wound is a unique problem in the treatment of burn patients since the length of stay of the patient in a hospital correlates to time of closure of the open wounds (1). Although different approaches have been attempted (2,3), closure of thermally injured skin with the patient's own skin, i.e. autografting or autologous grafting is currently the most common method (4,5). Typically, slices of skin being 0.008–0.012 inches thick are harvested from the patient's intact skin. Until the donor site wound itself is re-epithelialized, it is a breach in the integrity of the patient's skin. Frequently in major burn injuries that cover more than 30–40 % of the patient's total surface area repeated skin-harvesting procedure at the same donor site have to be performed and the rate of donor wound regeneration becomes the rate limiting factor for burn wound closure. Therefore, the faster the donor site heals, the more often grafting surgeries can be performed, consequently leading to improved outcome, decreased length of hospitalization and lower the risks of infectious complications (6,7,8). Although the significance of donor site wound healing is well recognized, few clinical studies have attempted to measure the process (9–13). For the most part, the rate of donor site wound healing and the effect of different therapeutic interventions on it have been studied using semi quantitative methods, such as histo- and immunohistochemical methods (9–11).
Synthesis of proteins is fundamental to the process of restoration of the wounded skin. Recently, we have developed new methods, based on stable isotope techniques, to quantify rates of protein turnover in wound and intact skin (12,13,14,15,16). So far the use of this method to measure skin/wound protein net balance has been successfully implemented in animal models (1-14-16). To our best knowledge there are only two articles that quantitatively report on protein deposition in regenerating wound in humans (12,13). Sakurai et al. (12) and Gore et al.(13), using stable isotope technique measured skin donor site and burn wound healing rates in adult burn patients. However, there is no data in the pediatric population.
Therefore, the purpose of this study was to determine donor site wound protein fractional synthesis rate (FSR) in pediatric burn patients using stable isotope technique and to see how the protein synthesis relates to the total body surface area burns (TBSA) and overall length of stay (LOS) in the pediatric patients in the intensive care unit (ICU).
METHODS
Patients
The study was conducted at the Shriners Hospital for Children in Galveston, TX, as a part of an ongoing research project. The study protocol was approved by the Institutional Review Board of the University of Texas Medical Branch. Informed child assent and/or parental permission were obtained from each patient and parent or guardian before study enrollment. Patients under 18 years, either sex, and with greater than 30 % total body surface area (TBSA) burn requiring skin grafting were eligible for the study.
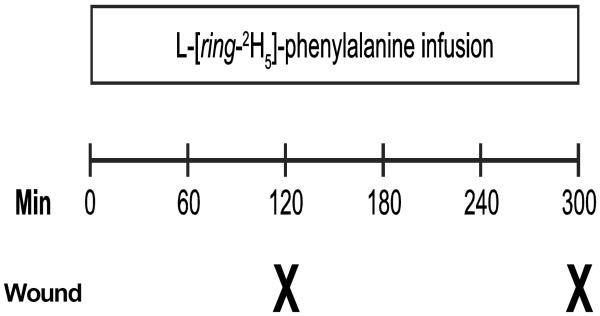
During burn wound autografting skin was harvested using an electric dermatome set at 0.008–0.012 inches (Zimmer Inc, Warsaw, IN). The donor site was dressed with Scarlet Red (Sherwood Medical, St. Louis, MO) impregnated fine mesh gauze. In 2 to 6 days after their grafting surgery the subjects underwent a 5-hour stable isotope infusion study with ongoing feeding (Vivonex). The metabolic study consisted of a primed (6.4 μmol/kg) continuous (0.16 μmol/kg/min) infusion of L-[ring-2H5]-phenylalanine (Cambridge Isotopes, Andover, MA) (Figure 1). Punch biopsies of the donor site wound were obtained at 120 and 300 min after initiation of the tracer infusion (Figure 1). The timing for the metabolic study was coordinated with clinically required graft staple removal procedure. Therefore, patients were given clinically necessary Midazolam (0.5 mg/kg, PO) and Ketamin (1 mg/kg, IV) and subcutaneous injection of 1% Lidocaine prior obtaining tissue biopsy followed by staple removal procedure. Donor site wound biopsies were obtained using 3 mm biopsy punch needles (Sklar Tru-Punch, Sklar Inst., PA). The samples were immediately frozen in liquid nitrogen for further analysis.
Figure 1.
Isotope infusion protocol. In the continuously fed state primed constant L-[ring-2H5]-phenylalanine isotope infusion was given for five hours. Donor site wound biopsies were performed under sterile condition and anesthesia using skin punch biopsy needles at 120 and 300 min after start of the infusion.
The primary outcome of the study was measurement of donor site wound protein FSR and secondary outcome was correlation of FSR with total burn surface area burns (TBSA); length of acute hospital stay (LOS) in ICU; LOS normalized for the TBSA and time after donor site wound creation or postoperative day when the metabolic study was conducted. Patients are normally discharged from the ICU when 95 % of initial total burn surface area has healed.
Sample processing
Twenty to twenty-five mg of wound was homogenized twice in 800 μL of 10% perchloric acid. The free intracellular enrichment of phenylalanine was measured from the supernatant after tissue homogenization and centrifugation. The bound phenylalanine incorporated into wound protein was measured from the pellet obtained after centrifugation. The pellet was washed and dried, and the proteins were hydrolyzed in 6 N HCl at 110°C for 24 hours (18). Amino acids were extracted with cation-exchange chromatography, and enrichment was determined after derivatization to tert-butyldimethylsilyl by gas chromatography-mass spectrometry (GC-MS HP 5989; Hewlett-Packard, Palo Alto, CA) with electron impact ionization. Ions 234 and 239 were monitored (18).
Calculations
FSR calculations were based on precursor-product method, using intracellular free and bound protein tracer enrichments in the wound biopsy as precursor and product, respectively. The following equation (18) was used for the calculations:
where Ef and Es are the enrichments of free and bound amino acid respectively, and t is the time interval in min between two sequential biopsies. FSR was expressed in percent per day (%/day).
Statistics
Values are presented Means±SD. Correlation analyses were performed by examining the Pearson Product moment. Multiple regression analysis was used to determine the relationship of estimated LOS/TBSA to other variables such as postsurgery day when the metabolic study was conducted and donor site wound protein FSR. A p value less than 0.05 was considered statistically significant.
RESULTS
Demographic parameters are presented in Table 1. This study is a part of larger study on the evaluation of anabolic agents in burn care. These patients were randomized to the control group and received only standard of care during acute burn hospitalization.
Table 1.
Demographics and parameters.
| Parameter | |
| Number of patients | 11 |
| Age, years | 8±6 |
| Sex (F/M) | 5/6 |
| Weight at admission, kg | 41±34 |
| Weight during the study, kg | 34±29 |
| TBSA burned (%) | 56±12 |
| Third degree (%) | 45±20 |
| LOS (days) | 30±15 |
| LOS/TBSA | 0.52±0.2 |
| Burn cause | |
| - Flame | 8 (73 %) |
| - Electric/Flame | 1 (9 %) |
| - Scald | 2 (18 %) |
| Donor site location | |
| - Thigh | 14 (77.8 %) |
| - Shoulder | 1 (5.6 %) |
| - Suprapubic area | 1 (5.6 %) |
| - Knee/pretibial area | 2 (11.1 %) |
Data presented as Mean±SD. TBSA, total body surface area; LOS, length of stay.
Data from eighteen metabolic studies from eleven patients were analyzed. Seven patients participated in the study twice to have a second time point of evaluation. Time interval between the two studies in these patients was 9±6.9 days.
The patients were admitted to our hospital on average 2.2±1.1 days postinjury.
Medical care was determined by faculty surgeons, fellows and residents according to clinical protocols that have been previously described (17).
Patients were fed with Vivonex (Sandoz Nutritional Corp., Minneapolis, MN) at 1.2 times their measured resting energy expenditure. The resting metabolic rate was determined once a week in the early morning at 30°C with a V-max 29 metabolic cart (Sensormedics, Yorba Linda, CA). The patients received nutritional supplements including a multi-vitamin, folic acid, zinc and vitamin C.
Donor site locations are presented in Table 1. The thigh was used in 78 % of cases.
The stable isotope infusion studies were conducted 3.9±1.1 days after grafting surgery. Donor site wound FSR was 10.4±7.5 %/day. This number means that about 10 % of donor site wound protein is synthesized per day (18).
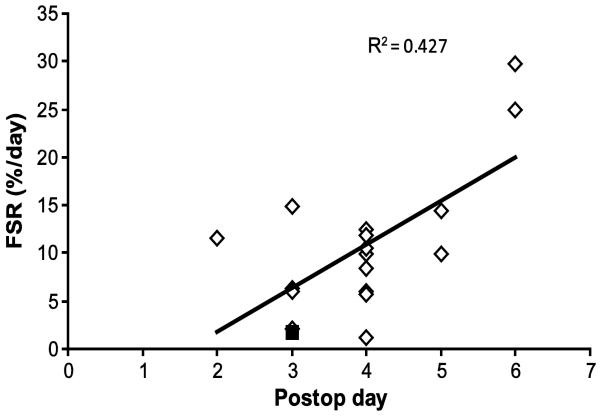
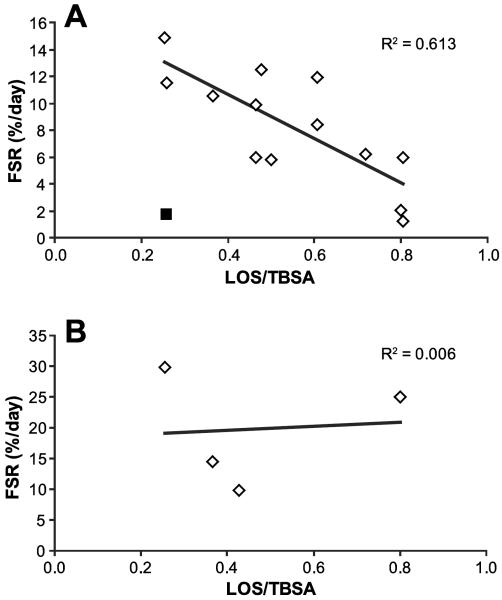
There was a significant correlation between time postsurgery and donor site wound protein FSR (p < 0.05) (Figure 2). Based on this result and the facts that there is almost ten-fold difference between skin dermal and epidermal protein FSR (15) and that at the later stages of donor site wound healing there is a defined epithelial layer (11) we conducted correlation analysis between FSR values and LOS/TBSA separating results into two periods depending on the postoperative day when the metabolic study was performed. In the “Early” period metabolic studies were performed at 2 to 4 days postsurgery, and in the “Late” period the studies were done on postsurgery days 5 and 6 (Figure 2). Regression analysis showed that wound protein FSR in the “Early” period significantly correlated with LOS/TBSA (p < 0.05) with r2 = 0.613 (Figure 3A). However, FSR of the “Late” period did not demonstrate any correlation with LOS/TBSA (p = 0.27) (Figure 3B).
Figure 2.
Donor site wound protein Fractional Synthesis Rate (FSR) by postsurgery (postop) days when the metabolic study was conducted, p < 0.05. One result was excluded from analysis (■) due to analytical problems.
Figure 3.
Association between donor site wound protein Fractional Synthesis Rate (FSR) and length of stay (LOS) normalized to total body surface area burns (TBSA). A. FSR measured at postsurgery days 2–4, p < 0.05. B. FSR measured at postsurgery days 5–6, p = 0.271. One result was excluded from analysis (■) due to analytical problems.
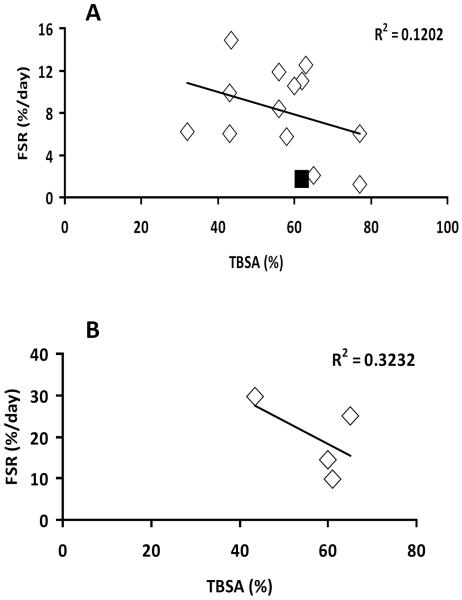
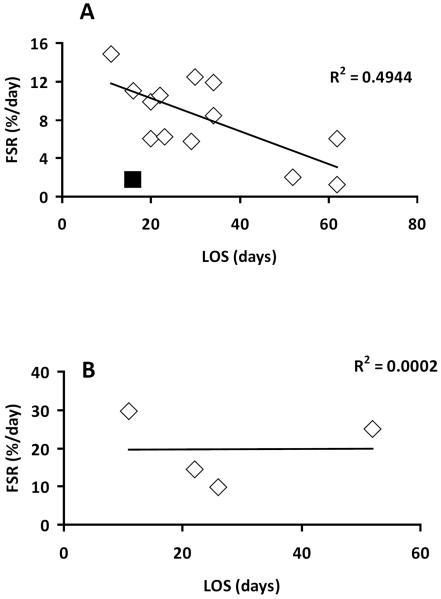
Furthermore, we evaluated if FSR correlates independently with either LOS or TBSA. There was no significant correlation (p > 0.05) between FSR and TBSA in either periods - “Early” and “Late” (Figure 4). In case of relationship with LOS, regression analysis determined a significant positive relationship (p < 0.05) between FSR and LOS in the “Early” period (Figure 5A), with r2 = 0.494. There was no correlation (p > 0.05) between FSR and LOS in the “Late” period (Figure 5B).
Figure 4.
Association between donor site wound protein Fractional Synthesis Rate (FSR) and total body surface area burns (TBSA). A. FSR measured at postsurgery days 2–4, p = 0.24. B. FSR measured at postsurgery days 5–6, p = 0.61. One result was excluded from analysis (■) due to analytical problems.
Figure 5.
Association between donor site wound protein Fractional Synthesis Rate (FSR) and length of acute hospital stay (LOS). A. FSR measured at postsurgery days 2–4, p < 0.05. B. FSR measured at postsurgery days 5–6, p = 0.96. One result was excluded from analysis (■) due to analytical problems.
In the “Early” group one result (FSR = 1.68 %/day) was excluded from correlation analysis (Figure 2A) because assessment of GCMS raw data revealed that the intracellular free enrichment of the tracer was three times higher than physiologically expected, which demonstrates that there was an analytical problem. An error of this nature would cause FSR to be underestimated three-fold, which would bring this patient's data into line with the other patients' data. Exclusion of any other FSR results did not result in any changes in the correlation or regression analyses.
The coefficient for the postsurgery day term was positive (Figure 2) whereas the coefficient for the LOS/TBSA (Figure 3A) term was negative; indicating that wound FSR was greater at later days after donor site wound creation, but lower for patients who required a longer length of stay.
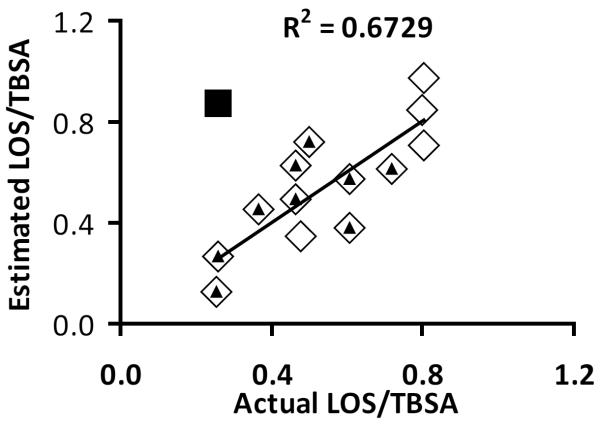
Multiple regression analysis showed that time postsurgery and LOS/TBSA significantly correlates with wound protein FSR (p < 0.001). Based on data from the regression analysis we derived the following formula to calculate estimated LOS/TBSA for the “Early” period: Estimated LOS/TBSA = (FSR - 12.95 – 1.414 × Postsurgery day)/(−17.8). Using this equation we calculated estimated values of LOS/TBSA for our study subjects and thereafter ran correlation analysis vs. actual LOS/TBSA (Figure 6). Correlation analysis demonstrated that donor site wound FSR corrected for the postsurgery day when the study was conducted accounted for 67 % of the variability (r2 = 0.673) in the actual LOS/TBSA (Figure 6). The linear regression analysis demonstrated a significant relationship (p < 0.001) between FSR corrected for the postsurgery day and actual LOS/TBSA.
Figure 6.
Correlation analysis of estimated LOS/TBSA (length of stay normalized for total body surface area burns) versus actual LOS/TBSA, p < 0.001, for the “Early” group, (◊), all results. Estimated LOS/TBSA was calculated by the following formula derived from multiple linear regression analysis to determine the relationship between donor site wound fractional synthesis rate (FSR) and postsurgery day when the metabolic study was conducted and actual LOS/TBSA: Estimated LOS/TBSA = (FSR - 12.95 - 1.141 × Postsurgery day) / (−17.8). The linear regression analysis demonstrated a significant correlation (p < 0.001) between the parameters. One result was excluded from analysis (■) due to analytical problems. The (▲) indicates results from the thigh, which composes 78 % of samples.
DISCUSSION
In this study, we measured the fractional synthesis rate (FSR) of protein in donor site in a burn pediatric patient population using a stable isotope technique. We found that donor site wound protein FSR gradually increases over time postsurgery and there is a correlation between donor site wound protein FSR and LOS or LOS/TBSA (Figure 2–4).
Presently the patients own skin is the preferred material to cover burn wounds (4,5). In severe burns availability of donor site for skin harvesting is limited and frequently the same donor site is used multiple times. This leads to numerous cycles of autograft harvesting and donor site healing. Several studies have evaluated effects of different pharmaceutical interventions on donor site wound healing and time period between grafting surgeries, and consequently LOS (9–13). However, evaluation methods were mostly observational (9–11) with only one quantitative study using stable isotope techniques (12).
Stable isotope techniques allow quantitative measurement of rates of protein synthesis and breakdown (18). Recently, our group developed and successfully implemented a new method for in vivo measurement of wound protein deposition using stable isotopes (14–16). Using this method, Zhang et al. (14) demonstrated that in rabbit donor site wound protein FSR increases over time, ranging from 6.6 to 20.5 %/day from postsurgery days 3 to 7, respectively. Our current data shows that the rate of protein synthesis increases over time in humans as well (Figure 2). This is probably related to distinct healing phases. 3–4 days old wounds are known to be completely re-epithelized with developed stratum basale though not fully differentiated and matured (19,20). Subsequent differentiation manifests by keratinization and covering with the stratum corneum. Collagen is the main component of the stratum basale and keratin the main component of stratum corneum. Therefore we speculate that on days 3–4 postsurgery we are measuring mostly FSR of collagen in stratum basale as well as dermis, and later a mixture of collagen and keratin synthesis. The work of Zhang et al. (15) examining differences in protein FSR in dermis and epidermis supports this hypothesis. One of the limitations of the current study is that we consider regenerating wound as a homogenous tissue, whereas it is composed of many proteins organized into different layers – dermis and epidermis. Currently, it is not feasible to measure the rate of synthesis of individual wound proteins in the clinic due to the large amount of tissue needed for the isolation procedure (e.g., about 200 mg for tropocollagen extraction, Dr. H. Kobayashi, personal communications).
With recent advances in treatment of burn injury in the acute phase and a consequent decrease in mortality rate LOS has become one of the main outcome measurements to reflect incidence of complications and treatment costs (21). Therefore, in this study we conducted a correlation analysis with LOS, TBSA, the main determining factor for LOS (22) and LOS normalized for TBSA. The analyses demonstrated that donor site wound healing is a strong predictor for LOS and LOS/TBSA, but does not have any relationship with TBSA. However, the correlation with LOS and LOS/TBSA was true only for the “Early” period. Normalization of LOS for TBSA has significantly improved r2, from 0.49 (FSR vs. LOS) to 0.61 (FSR vs. LOS/TBSA).
One of the possible limitations of this study is that the studies were conducted in the range of 2–6 days after donor site creation (3.9±1.1 days). This is explained by the flexible design of the study in order to coordinate the tracer studies with the clinical care. Although it would have been ideal to conduct all these studies on the same post donor site creation day there were beneficial sides of the current study design. First of all, we obtained scientifically new data showing that in humans donor site wound protein FSR increases over time post donor site creation. Secondly, the correlation between donor site wound protein FSR at “Early” but not “Late” period and acute LOS/TBSA was established (Fig. 3). The validity of the division of the studies into “Early” and “Late” periods was based on the previous histochemical and stable isotope studies (10–12,14), demonstrating the differences in time-dependent wound morphology.
In our study population LOS/TBSA ranged from 0.21 up to 0.81 days/%TBSA, and we were able to correlate it with donor site wound protein FSR (Figure 2A). It is interesting to note that in this study the two patients with the lowest FSR values (1.2 and 2.04 %/day) had the highest LOS/TBSA values - 0.81 and 0.80 days/%TBSA, respectively. Additionally, both of them had the highest number of grafting procedures – 11 and 10, respectively, (number of surgeries ranged from 2 to 11), and one of them had been diagnosed with sepsis that had lasted ten days prior to the metabolic study, supporting the clinical observation that the medical wellbeing of the patient has a significant impact on wound healing rate. However, it is preliminary to conclude whether infection or sepsis affects donor site wound protein FSR from this small number of studies. Further studies would be of interest to elaborate this part.
Multiple regression analysis showed that donor site wound protein FSR correlated with LOS/TBSA and postsurgery day when the metabolic study was conducted (p < 0.05). Based on data from the regression analysis we derived a formula to calculate estimated LOS/TBSA for our study subjects, thereafter we run correlation analysis vs. actual LOS/TBSA (Figure 4). The correlation analysis showed that donor site wound protein FSR corrected for the postsurgery day when the metabolic study was conducted accounted for 67 % (r2 = 0.673) of the actual LOS/TBSA. This correlation between the result of an analytical measurement and clinical outcome supports that the method we used in the current study can give reliable results that can be used in medical research.
Thigh was a donor site location in 78 % of studies, however including donor site location as an independent variable in multiple regression analysis did not affect the results.
In this study only seven patients participated in the metabolic study twice. From the other four patients, three patients' burn wounds healed before the second metabolic study could be scheduled, and the fourth patient did not have donor site generated during the first surgery. However, even if we evaluate the two studies with the same patients as separate studies denoted as “study 1” and “study 2”, there were still significant correlations between FSR and LOS/TBSA - “study 1”, r2 = 0.736 and “study 2”, r2 = 0.464.
Previously our laboratory reported FSR of protein in donor site in adult burn patients (12). Our current results are lower than the previously reported numbers. One explanation might be that the study populations are different – adults vs. children. It has been reported that there are dramatic differences in plasma cytokine profiles between adult and pediatric burn patients (23), and it is well known that cytokines actively participate in the wound healing process (24). However, further studies to test this hypothesis are of interest.
We used isotopic phenylalanine as the tracer amino acid because phenylalanine is not known to be oxidized or synthesized de novo in tissues except liver; therefore it is a reliable marker of the overall protein synthesis/kinetics in this model (18). The tracer incorporation method is based on the so called precursor-product principle, in which the change in degree of tracer incorporation into the tissue protein relative to the intracellular free tracer pool over time gives a value of the fractional rate of protein synthesis (18). There are two main assumptions underlying the model (18). Firstly, that an isotopic and physiologic steady state exists and secondly, intracellular free enrichment accurately reflects the true precursor enrichment. The first assumption is confirmed by consistent enrichment of the intracellular free tracer over the course of the study (120 and 300 min) (Data not shown). Additionally, it was previously reported that the intracellular free enrichment accurately reflects the true precursor enrichment (aminoacyl-tRNA) regardless of the amino acid tracer (25).
In conclusion, we found that the rate of protein synthesis in regenerating donor site wounds of burn patients increases over time postsurgery or post donor site creation. And, that at the early stages of wound healing it correlates to LOS or LOS/TBSA in the ICU. The current study is a small scale study that gives a good first approximation of relation between donor site wound healing and clinical outcome during acute burn treatment. Although not practical for routine clinical assessment, the novel stable isotope method used in this study may be used to assess the efficiency of therapeutic interventions that attempt to regenerate wounds faster in clinical research settings. Additionally, it may be used in clinical research involving skin protein metabolism in other pathological conditions. Examples include hypertrophic scarring, where it is known that there is an accelerated protein deposition in the skin matrix (26) or diabetic ulceration where it is known that the tissue degradation is accelerated (27). To our best knowledge, in neither condition skin protein turnover has been quantified so far. Therefore, the method opens new perspectives in the different fields of medical research that involve skin protein metabolism.
Acknowledgements
The authors wish to thank the study volunteers and their families for their patience and dedication, and the clinical and research staff of Shriners Hospital for Children, Galveston, TX for their help with conducting the clinical portion of the study; Tara Cocke, Christopher Danesi, Ann Hightower and Cindy Locklin for excellent technical performance, and Guy Jones and Jariwala Guarang for performing GCMS analyses.
This study was supported by grants: NIGMS T32-GM08256, NIGMS P50-GM60338, NIGMS R01-GM56687, NIDRR H133A020102 and Shrine grants 8490, 8660, 8760, 8570, 8600.
REFERENCES
- 1.Herndon DN, Barrow RE, Rutan RL, Desai MH, Abston S. A comparison of conservative versus early excision: Therapies in severely burned patients. Ann Surg. 1989;209:547–553. doi: 10.1097/00000658-198905000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413–428. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce ST, Kagan RJ, Yakuboff KP, Meyer NA, Reiman MT, Greenhalgh DG, Warden GD. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann Surg. 2002;235:269–279. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kogan L, Govrin-Yehudain J. Vertical (two-layer) skin grafting: new reserves for autologic skin. Ann Plast Surg. 2003;50:514–516. doi: 10.1097/01.SAP.0000044150.03940.4E. [DOI] [PubMed] [Google Scholar]
- 5.Archer SB, Henke A, Greenhalgh DG, Warden GD. The use of sheet autografts to cover extensive burns in patients. J Burn Care Rehabil. 1998;19:33–38. doi: 10.1097/00004630-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Desai MH, Herndon DN, Broemeling L, Barrow RE, Nichols RJ, Rutan RL. Early burn wound excision significantly reduces blood loss. Ann Surg. 1990;211:753–762. doi: 10.1097/00000658-199006000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke JF, Quinby WC, Bondoc CC. Early excision and prompt wound closure supplemented with immunosuppression. Surg Clin North Am. 1978;58:1141–1150. doi: 10.1016/s0039-6109(16)41682-8. [DOI] [PubMed] [Google Scholar]
- 8.Pietsch JB, Netscher DT, Nagaraj HS, Groff DB. Early excision of major burns in children: effect on morbidity and mortality. J Pediatr Surg. 1985;20:754–757. doi: 10.1016/s0022-3468(85)80039-7. [DOI] [PubMed] [Google Scholar]
- 9.Herndon DN, Barrow RE, Kunkel KR, Broemeling L, Rutan RL. Effects of recombinant human growth hormone on donor-site healing in severely burned children. Ann Surg. 1990;212:424–429. doi: 10.1097/00000658-199010000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilpin DA, Barrow RE, Rutan RL, Broemeling L, Herndon DN. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Ann Surg. 1994;220:19–24. doi: 10.1097/00000658-199407000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierre EJ, Barrow RE, Hawkins HL, Nguyen TT, Sakurai M, Desai A, Wolfe RR, Herndon DN. Effects of insulin on wound healing. J Trauma. 1998;44:342–345. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguen TT, Patterson BW, Wolfe RR. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:283–297. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. J Parenter Enteral Nutr. 2006;30:331–338. doi: 10.1177/0148607106030004331. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XJ, Chinkes DL, Cox RA, Wolfe RR. The flow phase of wound metabolism is characterized by stimulated protein synthesis rather than cell proliferation. J Surg Res. 2006;135:61–67. doi: 10.1016/j.jss.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XJ, Chinkes DL, Wolfe RR. Measurement of protein metabolism in epidermis and dermis. Am J Physiol Endocrinol Metab. 2003;284:E1191–E1201. doi: 10.1152/ajpendo.00460.2002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XJ, Chinkes DL, Wolfe RR. Leucine supplementation has an anabolic effect on proteins in rabbit skin wound and muscle. J Nutr. 2004;134:3313–3318. doi: 10.1093/jn/134.12.3313. [DOI] [PubMed] [Google Scholar]
- 17.Pereira C, Murphy K, Jeschke M, Herndon DN. Post burn muscle wasting and the effects of treatments. Int J Biochem Cell Biol. 2005;37:1948–1961. doi: 10.1016/j.biocel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research. John Wiley & Sons, Inc., publication; New Jersey: 2005. [Google Scholar]
- 19.Viziam CB, Matoltsy AG, Mescon H. Epithelialization of small wounds. J Invest Dermat. 1964;43:499–507. [PubMed] [Google Scholar]
- 20.Odland G, Ross R. Human wound repair. J Cell Biol. 1968;39:135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrell SW, Saffle JR, Sullivan JJ, Larsen CM, Warden GD. Increased survival after major thermal injury. A nine year review. Am J Surg. 1987;154:623–627. doi: 10.1016/0002-9610(87)90229-7. [DOI] [PubMed] [Google Scholar]
- 22.Andel D, Kamolz LP, Niedermayr M, Hoerauf K, Schramm W, Andel H. Which of the abbreviated burn severity index variables are having impact on the hospital length of stay? J Burn Care Res. 2007;28:163–166. doi: 10.1097/BCR.0B013E31802C9E8F. [DOI] [PubMed] [Google Scholar]
- 23.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D, Tompkins RG. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14:553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirsner RS, Bogensberger G. The normal process of healing. In: Kloth LC, McCulloch JM, editors. Wound healing alternatives in management. F.A.Davis company; Philadelphia: 2002. pp. 3–34. [Google Scholar]
- 25.Martini WZ, Chinkes DL, Wolfe RR. The intracellular free amino acid pool represents tracer precursor enrichment for calculation of protein synthesis in cultured fibroblasts and myocytes. J Nutr. 2004;134:1546–50. doi: 10.1093/jn/134.6.1546. [DOI] [PubMed] [Google Scholar]
- 26.Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids, and contractures: The cellular and molecular basis for therapy. Surg. Clin. North Am. 1997;77:701–730. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen WY, Roggers AA. Recent insights into the causes of chronic ulceration in venous disease and implications on other types of chronic wounds. Wound Repair Regen. 2007;15:434–449. doi: 10.1111/j.1524-475X.2007.00250.x. [DOI] [PubMed] [Google Scholar]