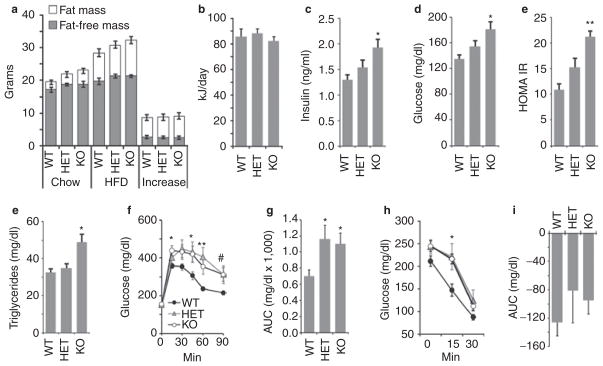
Figure 5.
Adropin-deficiency does not affect weight gain associated with high-fat diets, but is associated with an increased severity of impaired glucose homeostasis associated with obesity. (a) Body composition of wild-type control (WT), heterozygous carriers of the null adropin allele (HET) and adropin knockout (KO) mice weaned onto chow, and then after 8 weeks on the high-fat diet (60% kJ/fat, HFD) (n = 6/group). Exposure to the HFD resulted in weight gain predominantly due to increased fat mass. Body weight and composition were not significantly different after 8 weeks on HFD; at the start of the study, there was a tendency (P = 0.07) for increased fat mass in adropin knockout mice. (b) Food intake expressed as kJ/day was not significantly affected by genotype. Food intake was recorded over a 1 month period. (c–e) Insulin and glucose measurements recorded at the end of 8 weeks on HFD. Hyperinsulinemia and hyperglycemia were more severe in adropin knockout mice relative to WT. The differences in insulin, blood glucose and homeostasis model assessment–insulin resistance (HOMAIR) between WT and KO were significant (*P < 0.05, **P < 0.001). The differences in insulin, blood glucose, and HOMAIR between HET and KO were not statistically significant. (e) Adropin knockout mice exhibited fasting hypertriglyceridemia (*P < 0.05 vs. WT). (f, g) Impaired glucose tolerance associated with diet-induced obesity is more severe in heterozygous and homozygous carriers of the null adropin allele. Shown are the raw data (f, P < 0.05 when comparing WT vs. *KO only, **WT vs. KO and HET, #WT vs. HET only) and the area under the curve (AUC) above baseline levels (g, *P < 0.05 vs. WT). (h, i) Insulin tolerance tests suggest reduced insulin action in AdrKO carriers of the null adropin allele fed high-fat diet. *P < 0.05, WT vs. KO.